Circulating biomarker for malignant pleural mesothelioma diagnosis: pay attention to study design
Like the majority of malignant diseases, malignant pleural mesothelioma (MPM) is usually diagnosed on the basis of biopsy findings (1). However, biopsy is an invasive approach, and its accuracy is greatly affected by the quality of the specimen and the experience of observers. Imaging approaches are also used to assist the diagnosis of MPM, but the inter-observer variation is also a problem. Therefore, non-invasive and objective tests, such as circulating tumor markers, are of great value for MPM diagnosis (2).
Before a biomarker being widely used in clinical practice, rigorous analytical validations must be performed to confirm whether the analytical method is robust. Besides, clinical studies should be performed to evaluate the diagnostic accuracy of the biomarker for a target disease. If the analytical method is robust and the diagnostic value is acceptable, the tumor marker may be approved by Food and Drug Administration (FDA) for the diagnosis of a target cancer (3). Every year, many biomarkers were reported by translational researches; however, only small portion of these biomarkers were further validated by clinical studies (4), and the probability of being approved by FDA was extremely low. This might be due to the test method for the biomarker was not reliable, or the clinical studies revealed that the diagnostic accuracy of the biomarker is limited (5). The clinical validation is a crucial step for the development of a biomarker, as it will greatly determine the fate of a biomarker.
During past years, remarkable efforts have been made to explore the promising circulating diagnostic markers for MPM. However, only small portion of biomarkers have been widely validate to date (2,6,7), such as soluble mesothelin-related peptides (SMRP). It is noteworthy that the majority of the available studies had design weaknesses, especially in subject selection, which may bias the results.
The major principal for patient selection in a study of diagnostic accuracy is “real world”. Which means the characteristics of the subjects in the study must be inconsistent with those of target population in clinical practice. Target population is usually defined as the subjects in whom a certain disease is suspected. For MPM, the target population may be defined as patients who have the following characteristics: (I) chronic chest pain or cough; (II) history of asbestos-exposure; (III) chronic chest pain or cough with no prima reason. Actually, these characteristics are usually expressed as inclusion or exclusion criteria in a study. Among the available studies that investigating the diagnostic accuracy of a tumor marker for MPM, some of them lack of pre-specified inclusion or exclusion criteria. The authors only reported the sample sizes of MPM and controls their enrolled, which is termed “two gate-design”. In a two-gate design study, the clinical characteristics of the subjects, especially the severity of the disease may not be consistent with that in clinical practice (8); therefore, the conclusion of the study may not be generalized to clinical practice.
Another weakness of two-gate design is that the prevalence of target disease (MPM) in study cohort may not be inconsistent with that in “real world”. Theoretically, sensitivity and specificity are not affected by the prevalence of target disease in study cohort, if they the subjects were separately enrolled in a random or consecutive manner. However, negative and positive predictive values (NPV and PPV) are great affected by prevalence. Compared with sensitivity and specificity, PPV and NPV are more clinically meaningful because their interpretations are more straightforward (9). For example, for a suspected patient with positive SMRP, we need to know the probability that the patients will be diagnosed as MPM. While the sensitivity and specificity do not give us this information.
For instance, in a target population which is defined by the pre-specified criteria, the prevalence of MPM is 20%. That means, if 500 suspected MPM patients were consecutively enrolled, 100 of them would be diagnosed as MPM and the remaining 400 were non-MPM. We supposed that both the sensitivity and specificity of SMRP for MPM is 0.80, a 2 by 2 table can be constructed, as shown in Table 1. Obviously, the PPV in cohort study is 0.50, indicating that if a patient had a positive SMRP, the probability of MPM is 50%.
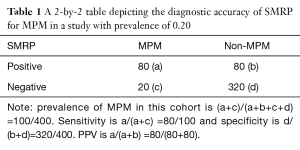
Full table
In a two-gate design study, consecutive enrollment of study cohort is impossible, therefore, the prevalence of MPM in study cohort may be not consistent with that in “real world”. We suppose that the prevalence of MPM in a two-gate design study is 0.50, as shown in Table 2. Although the sensitivity and specificity are not affected, the PPV is increased to 0.80, indicating that if a suspected MPM patients had positive SMRP, the probability of MPM is 80%. Therefore, the PPV may be over- or underestimated in studies with two-gate design.
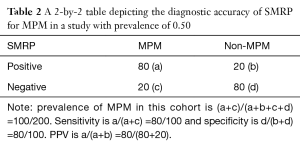
Full table
Of note, the inclusion and exclusion criteria may vary in different studies, and the prevalence of MPM in study cohort is varied correspondingly. Therefore, in a meta-analysis, subgroup analysis based on inclusion and exclusion criteria should be performed to reveal the diagnostic accuracy of an index test across cohorts with difference characteristics.
Taken together, one-gate design and consecutive enrolment are crucial to keep the characteristics of study cohort being inconsistent with “real world”, and therefore, the conclusion of the study can be generalized to clinical practice. Noteworthy, majority of available studies investigating the diagnostic accuracy of tumor markers for MPM is two-gate design, therefore, their conclusions may not be reliable to be generalized to clinical practice. Further studies with one-gate design and consecutive enrollment are needed to rigorously evaluate the diagnostic value of tumor markers for MPM.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Opitz I. Management of malignant pleural mesothelioma-The European experience. J Thorac Dis 2014;6 Suppl 2:S238-52. [PubMed]
- Panou V, Vyberg M, Weinreich UM, et al. The established and future biomarkers of malignant pleural mesothelioma. Cancer Treat Rev 2015;41:486-95. [Crossref] [PubMed]
- Wang Y. Development of cancer diagnostics—from biomarkers to clinical tests. Transl Cancer Res 2015;4:270-9.
- Goossens N, Nakagawa S, Sun X, et al. Cancer biomarker discovery and validation. Transl Cancer Res 2015;4:256-69. [PubMed]
- Chen JJ, Lu TP, Chen DT, et al. Biomarker adaptive designs in clinical trials. Transl Cancer Res 2014;3:279-92.
- Zhang W, Wu X, Wu L, et al. Advances in the diagnosis, treatment and prognosis of malignant pleural mesothelioma. Ann Transl Med 2015;3:182. [PubMed]
- Vigneri P, Martorana F, Manzella L, et al. Biomarkers and prognostic factors for malignant pleural mesothelioma. Future Oncol 2015;11:29-33. [Crossref] [PubMed]
- Rutjes AW, Reitsma JB, Vandenbroucke JP, et al. Case-control and two-gate designs in diagnostic accuracy studies. Clin Chem 2005;51:1335-41. [Crossref] [PubMed]
- Altman DG, Bland JM. Diagnostic tests 2: Predictive values. BMJ 1994;309:102. [Crossref] [PubMed]


