Editorial on the article entitled “brigatinib efficacy and safety in patients with anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer in a phase I/II trial”
Anaplastic lymphoma kinase (ALK)-rearrangements occur in 3–7% of patients with non-small cell lung cancer (NSCLC) and are more common among patients with a never/light smoking history, adenocarcinoma histology, youngers and in tumors wild-type for EGFR and KRAS genes (1-4). Crizotinib (Xalkori®; PF-02341066; Pfizer), a small molecule inhibitor of ALK, ROS1 and MET (5-7), was the first tyrosine kinase inhibitor (TKI) approved by the Food and Drug Administration (FDA) and the European Medicine Agency (EMA) for patients with NSCLC who have the ALK gene rearrangement, because it induces rapid tumor regression and objective responses around 70% in the majority of such patients, both in first and second line settings (8,9). Unfortunately, as occurred with other TKIs, the disease progressed within the first 12 months and the central nervous system (CNS) is one of the most common first sites of progression. Within different mechanisms of resistance, the emergence of acquired crizotinib-resistance mutations, such as the ‘gate-keeper’ L1196M, the G1269A, C1156Y and G1202R mutations, among others, are the main cause of crizotinib-resistant disease (10-15). In light of its limitations, several next-generation ALK-inhibitors were rapidly developed. Ceritinib (Zykadia™; LDK378; Novartis) was the first FDA and EMA drug approved for patients who have experienced progression on or are intolerant to crizotinib. Ceritinib has shown objective response rates (ORR) around 60% across its “ASCEND” development (16-19). Unlike crizotinib, ceritinib exhibited higher incidence of gastrointestinal adverse events. While the majority of crizotinib-resistant patients respond to ceritinib, acquired secondary resistance has been reported (20). Within the most developed second-generation ALK-inhibitors beyond ceritinib, alectinib (Alecensa™; CH5424802/RO5424802; Chugai-Roche), based on two phase II trials, was approved by the FDA: one was performed in a global population (21), and the other one was conducted in the United States and Canada (22). In addition, alectinib is the first ALK-inhibitor that has demonstrated superior efficacy against crizotinib in a head to head phase III trial. A multicenter randomized, open-label phase III trial (J-ALEX; JapicCTI-132316) was conducted in Japan by Nokihara and colleagues, to compare alectinib (300 mg twice daily) versus crizotinib (250 mg twice daily) in 207 ALK+ NSCLC patients who had received ≤1 prior chemotherapy regimen and no prior ALK-inhibitors. Of note, Japan regulates alectinib to a lower dose than does the rest of the world, which uses alectinib at 600 mg twice daily (23). Results from a pre-specified interim analysis showed an increased progression-free survival (PFS) by 66% compared with crizotinib [hazard ratio (HR) =0.34; 99% confidence interval (CI), 0.17–0.70, P<0.0001]. Median PFS was not reached in patients who received alectinib (95% CI, 20.3 months-not estimable) versus 10.2 (95% CI, 8.2–12.0) months in patients who received crizotinib. The ORR determined by an independent review favored alectinib (91.6% vs. 78.9%). Otherwise, alectinib reduced significantly the risk of progression by 92% compared with crizotinib in patients with brain metastases at baseline (HR =0.08; 95% CI, 0.01–0.61) (23). Moreover, alectinib is being compared with crizotinib in chemotherapy-naïve ALK+ NSCLC patients in the global phase III ALEX trial (NCT02075840). As it happens during crizotinib and ceritinib treatments, patients treated with alectinib develop resistance and progress invariably (13,24,25).
Taken into account that up to 50% of patients with ALK+ NSCLC develop CNS metastases during the course of their disease (26,27), is of extreme interest to promote the development of drugs with an increased blood-brain barrier penetration. Indeed, lorlatinib (PF-06463922; Pfizer), a third-generation ALK and ROS1 inhibitor, was specifically designed to increase tumor and CNS penetration by ensuring an increased lipophilicity and a decreased molecular weight (28). Among the 54 patients included in the phase I/II trial (NCT01970865), 39 patients (72%) had brain metastases at baseline. For patients with intra- and extra-cranial disease, the ORR was 50% (26/52 evaluable patients) and the intra-cranial ORR was 60% (12/20) in patients with target lesions (29).
Brigatinib (AP26113, Ariad) is a novel potent third-generation, orally available ALK-inhibitor, that appears to inhibit ALK-resistance mutations from first- and second-generation ALK-inhibitors, including G1202R, not yet approved for clinical use. Brigatinib also showed activity against ROS1 (30,31) and mutant EGFR, including T790M resistant mutation (32). Results from the ongoing phase I/II, single-arm, open-label, multicenter trial in advanced malignancies, including ALK+ NSCLC, has been reported by Rosell and colleagues (NCT01449461) (33). A total of 137 patients received brigatinib at once-daily doses of 30 to 300 mg. Safety was reported in all patients and efficacy in 78 out of 79 ALK+ NSCLC patients. Among 79 ALK+ NSCLC patients whose median age was 54 (range, 29–83) years, 49% were female, and 90% had previously received crizotinib. At the date of cut-off on February 17th, 2015, 45/79 (57%) patients remained on study and the median time on treatment was 12.6 months (range, 1 day to 35.5 months). The ORR was 74% (58/78 patients) (95% CI, 63% to 84%) with a median PFS of 13.4 months. Among 70 evaluable ALK+ NSCLC patients with prior crizotinib therapy, the ORR was 71% (50/70) (95% CI, 59% to 82%) compared to 100% (8/8) (95% CI, 63% to 100%) in the crizotinib-naïve subgroup, including 3 complete responses. The median PFSs were not reached in either subgroup. In a post hoc independent radiological review of patients with brain metastases (BM) at baseline, 8/15 (53%) with measurable lesions (≥10 mm) achieved an intracranial objective response with an intracranial disease control rate of 87%. The toxicity profile described in all 137 patients (all grades) was nausea (52%), fatigue (42%) and diarrhea (40%). Grade ≥3 treatment-related adverse events (AEs) included elevated lipase (9%), dyspnea (7%), hypertension (5%), hypoxia (5%), pneumonia (5%), elevated amylase (4%), fatigue (4%), pulmonary embolism (3%), elevated ALT (2%), hyponatremia (2%) and hypophosphatemia (2%) (33).
Kim and colleagues recently presented the first report on efficacy and safety of brigatinib in the pivotal randomized phase II trial (ALTA; NCT02094573) including 222 crizotinib-treated ALK+ NSCLC patients. Prior chemotherapy was allowed, and 180 patients (69%) had baseline brain metastases. Patients were randomized to brigatinib at 90 or 180 mg once daily. In the 180 mg arm, patients were started at 90 mg for 1 week before increasing to 180 mg. Patients in the 180 mg arm versus the 90 mg arm had superior ORR (54% vs. 45%), disease control rate (86% vs. 82%), and median PFS (12.9 vs. 9.2 months). The median overall survival (OS) was not reached in either arm, but the 1-year OS rate was higher in the 180 mg arm (80% vs. 71%; HR, 0.57). In addition to provide a better systemic efficacy, the 180 mg dose was associated with a higher intracranial response rate (67% vs. 36%), although the intracranial disease control rate was similar in the 180 mg and 90 mg arms (83% and 88%, respectively). Intracranial PFS was prolonged in the 180 mg arm (not reached vs. 15.6 months) (34). The toxicity profile of the 180 mg dose showed more grade 1 or 2 AEs. Brigatinib, at 90 mg and 180 mg was associated with (any grade) nausea (33%/40%), diarrhea (19%/38%), headache (28%/27%), fatigue (20%/27%), vomiting (24%/23%), and dyspnea (21%). The dose-reduction rate was higher in the 180 mg arm (20% vs. 7%), as well as the dose-interruption rate (36% vs. 18%). Six percent of patients experienced early-onset pulmonary adverse events (hypoxia, dyspnea, cough, pneumonia, pneumonitis), and 3% had grade ≥3 toxicity (34).
Unlike crizotinib, ceritinib and alectinib, to date, no single secondary ALK kinase domain mutations that confer primary or secondary resistance to brigatinib has been identified (35). Table 1 summarized the systemic and intracranial efficacies of second and third-generation ALK TKIs.
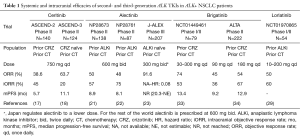
Full table
Based on the available single-arm studies of ceritinib and alectinib in crizotinib-resistant disease, alectinib appears with potential advantages over ceritinib, mainly in its higher intracranial activity and favorable toxicity profile. Despite the preliminary results from the Japanese J-ALEX trial (23), to date is unknown whether frontline alectinib improves survival outcomes compared with the strategy of frontline crizotinib followed by alectinib upon progression. While is true that the global (ALEX) trial of first-line alectinib vs. crizotinib could support the benefit of alectinib in this setting, the crossover was not included in the design, precluding a direct comparison of first-line alectinib vs. sequential crizotinib/alectinib. If alectinib become as the recommended ALK-inhibitor, the subsequent point is which third-generation ALK TKI should be prescribed beyond alectinib progression. Unfortunately, to date there are no randomized trials comparing next-generation ALK- inhibitors in the setting of crizotinib resistance, limiting our ability to compare these agents directly.
Brigatinib is of major interest, as it has been shown in preclinical models to target known first- and second-generation ALK-resistant mutations, and nowadays it has demonstrated in clinical trials high activity in crizotinib/ceritinib/alectinib-refractory ALK+ NSCLC patients. The ALTA trial demonstrated higher efficacy with the 180 mg daily dose, which will move forward for development. In general, brigatinib was well tolerated, but in a proportion of cases, early onset pulmonary events were seen in 14% (6/44) of patients treated at starting dose of 180 mg, but in only 4% (2/50) of patients who started at 90 mg (including patients escalating to 180 mg after one week of treatment). Digestive toxicity seems to be independent of the dose level. Indeed, grade ≥3 increased amylase and lipase were most common at 90 mg than 90 mg → 180 or 180 mg. Nausea only was reported in 4% of patients at 180 mg (33). The toxicity profile reported for lorlatinib included any-grade hypercholesterolemia (54%) and peripheral edema (37%) as the most frequent drug-related AEs. Hypercholesterolemia was the most common (9%) grade ≥3 AE and the most frequent reason for dose delay/reduction (29).
To date, based on the safety data reported in clinical trials, and due to the lack of head to head studies comparing new ALK-inhibitors, it is difficult to know which is the best treatment in first-line setting. If ALEX trial confirms the benefit on efficacy and tolerability of alectinib against crizotinib, it would become as a major option in first- line for ALK+ NSCLC. All of these drugs have demonstrated robust efficacy results treating CNS disease. The ongoing phase III ALTA-1L trial (NCT02737501) randomized patients to receive either brigatinib, 90 mg once daily for 7 days, then 180 mg once daily, or crizotinib, 250 mg twice daily. Crossover to brigatinib is allowed at crizotinib progression. This trial could clarify the optimal approach to targeting ALK in first-line treatment.
In summary, brigatinib may have a place in the sequence for ALK+ NSCLC targeting ALK-resistant mutations that crizotinib, ceritinib, and alectinib may not. Until now, the optimal sequence of ALK-inhibitors needs to be individualized according to the type of resistant mutations and the presence of CNS disease. In Figure 1 we propose an algorithm to treat patients with ALK+ NSCLC.
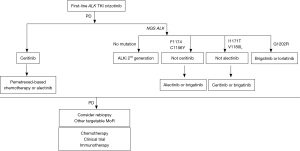
Acknowledgements
None.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Di Lu (Nanfang Hospital, Southern Medical University, Guangzhou, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009;115:1723-33. [Crossref] [PubMed]
- Takahashi T, Sonobe M, Kobayashi M, et al. Clinicopathologic features of non-small-cell lung cancer with EML4-ALK fusion gene. Ann Surg Oncol 2010;17:889-97. [Crossref] [PubMed]
- Camidge DR, Kono SA, Flacco A, et al. Optimizing the detection of lung cancer patients harboring anaplastic lymphoma kinase (ALK) gene rearrangements potentially suitable for ALK inhibitor treatment. Clin Cancer Res 2010;16:5581-90. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol 2011;6:942-6. [Crossref] [PubMed]
- Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012;30:863-70. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010;363:1734-9. [Crossref] [PubMed]
- Sasaki T, Okuda K, Zheng W, et al. The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res 2010;70:10038-43. [Crossref] [PubMed]
- Sasaki T, Koivunen J, Ogino A, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res 2011;71:6051-60. [Crossref] [PubMed]
- Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 2012;4:120ra17. [Crossref] [PubMed]
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472-82. [Crossref] [PubMed]
- Ignatius Ou SH, Azada M, Hsiang DJ, et al. Next-generation sequencing reveals a Novel NSCLC ALK F1174V mutation and confirms ALK G1202R mutation confers high-level resistance to alectinib (CH5424802/RO5424802) in ALK-rearranged NSCLC patients who progressed on crizotinib. J Thorac Oncol 2014;9:549-53. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Mok T, Spigel D, Felip E, et al. ASCEND-2: A single-arm, open-label, multicenter phase II study of ceritinib in adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) previously treated with chemotherapy and crizotinib (CRZ). J Clin Oncol 2015;33:abstr 8059.
- Felip E, Orlov S, Park K, et al. ASCEND-3: A single-arm, open-label, multicenter phase II study of ceritinib in ALKi-naïve adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:abstr 8060.
- Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452-63. [Crossref] [PubMed]
- Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 2014;4:662-73. [Crossref] [PubMed]
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661-8. [Crossref] [PubMed]
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234-42. [Crossref] [PubMed]
- Nokihara H, Hida T, Kondo M, et al. Alectinib (ALC) versus crizotinib (CRZ) in ALK-inhibitor naive ALK-positive non-small cell lung cancer (ALK+ NSCLC): Primary results from the J-ALEX study. J Clin Oncol 2016;34:abstr 9008.
- Ou SH, Klempner SJ, Greenbowe JR, et al. Identification of a novel HIP1-ALK fusion variant in Non-Small-Cell Lung Cancer (NSCLC) and discovery of ALK I1171 (I1171N/S) mutations in two ALK-rearranged NSCLC patients with resistance to Alectinib. J Thorac Oncol 2014;9:1821-5. [Crossref] [PubMed]
- Toyokawa G, Hirai F, Inamasu E, et al. Secondary mutations at I1171 in the ALK gene confer resistance to both Crizotinib and Alectinib. J Thorac Oncol 2014;9:e86-7. [Crossref] [PubMed]
- Camidge DR. Taking aim at ALK across the blood-brain barrier. J Thorac Oncol 2013;8:389-90. [Crossref] [PubMed]
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem 2014;57:4720-44. [Crossref] [PubMed]
- Solomon BJ, Bauer TM, Felip E, et al. Safety and efficacy of lorlatinib (PF-06463922) from the dose-escalation component of a study in patients with advanced ALK+ or ROS1+ non-small cell lung cancer (NSCLC). J Clin Oncol 2016;34:abstr 9009.
- Zhang S, Wang F, Keats J, et al. Abstract LB-298: AP26113, a potent ALK inhibitor, overcomes mutations in EML4-ALK that confer resistance to PF-02341066 (PF1066). 2010;70:abstr LB-298.
- Squillace RM, Anjum R, Miller D, et al. AP26113 possesses pan-inhibitory activity versus crizotinib-resistant ALK mutants and oncogenic ROS1 fusions. Cancer Research 2013;73:abstr 5655.
- Rivera VM, Wang F, Anjum R, et al. Abstract 1794: AP26113 is a dual ALK/EGFR inhibitor: Characterization against EGFR T790M in cell and mouse models of NSCLC. Cancer Research 2012;72:abstr 1794.
- Rosell R, Gettinger SN, Bazhenova LA, et al. 1330: Brigatinib efficacy and safety in patients (Pts) with anaplastic lymphoma kinase (ALK)-positive (ALK+) non-small cell lung cancer (NSCLC) in a phase 1/2 trial. J Thorac Oncol 2016;11:S114. [Crossref] [PubMed]
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib (BRG) in patients (pts) with crizotinib (CRZ)-refractory ALK+ non-small cell lung cancer (NSCLC): First report of efficacy and safety from a pivotal randomized phase (ph) 2 trial (ALTA). J Clin Oncol 2016;34:abstr 9007.
- Gettinger SN, Zhang S, Hodgson JG, et al. Activity of brigatinib (BRG) in crizotinib (CRZ) resistant patients (pts) according to ALK mutation status. J Clin Oncol 2016;34:abstr 9060.


