Angiopoietin-2: marker or mediator of angiogenesis in continuous-flow left ventricular assist device patients?
Recent advances in left ventricular assist device (LVAD) technology have revolutionized care of patients with end-stage heart failure (HF) both as bridge-to-transplantation and as destination therapy (1,2). Over the last decade, older generation pulsatile-flow LVADs (PF-LVADs) were replaced with new generation continuous-flow LVADs (CF-LVADs) due to their ease of implantation, improved durability, and vastly superior outcomes demonstrated in clinical trials. Although infectious complications were notably lower in patients supported with CF-LVADs as compared to PF-LVADs, non-surgical bleeding (NSB) complications—particularly gastrointestinal bleeding (GIB)—were significantly more prevalent in these patients leading to increased rates of hospital readmissions, blood transfusions, and gastrointestinal interventions. Interestingly, endoscopic studies have suggested that a significant proportion of GIB events in CF-LVAD patients are due to arteriovenous malformations (AVMs). Prior studies have suggested loss of pulsatility as well as acquired von Willebrand disease as potential contributors to this phenomenon (3,4). The mechanisms that underlie this deregulated angiogenesis in CF-LVAD patients, however, remain largely unknown to date.
Viewed in this context, the current Circulation article by Tabit and colleagues represents an important contribution to the field. In this cross sectional analysis of 101 patients, the authors analyzed angiopoietin-2 (Ang-2) as a marker of endothelial injury and as a possible mediator of angiogenesis in patients supported with CF-LVADs. In comparison to patients with HF or orthotopic heart transplantation (OHT), authors observed that CF-LVAD patients had significantly higher serum levels and endothelial expression of Ang-2. Moreover, serum from CF-LVAD patients led to greater endothelial tube formation in-vitro: an effect that was dampened by Ang-2 blockade. Importantly, the authors demonstrated that serum levels of thrombin, factors XIIa and XIa were also increased in patients supported with CF-LVADs, and that increased thrombin could be responsible for elevated Ang-2 expression, as treatment with a thrombin receptor antagonist reduced Ang-2 levels in endothelial cells treated with plasma obtained from CF-LVAD patients. Taken together, these findings suggest that thrombin is an upstream regulator of Ang-2 signaling in endothelial cells, and thrombin activation could be the underlying mechanisms for elevated Ang-2 observed in these patients.
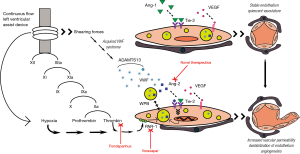
Before addressing the clinical significance of this study, it is useful to digress for a moment to discuss the biological plausibility of the Ang2-Tie axis on AVM formation in CF-LVAD patients. The focus of the manuscript is the angiopoietin-tie ligand-receptor system, which plays an instrumental role in maintaining vascular homeostasis. The primary ligands, angiopoietin-1 (Ang-1) and Ang-2, are key mediators in regulating the balance between vascular quiescence and instability, and thus are tightly regulated (Figure 1). Ang-1 is constitutively expressed in various cells types (e.g., pericytes, smooth muscle cells, fibroblasts) (5). When bound to the Tie-2 receptor, Ang-1 activates protein kinase B-Akt pathway, hence promoting survival of endothelial cells through inhibition of apoptosis (6-8). In this way, Ang-1 acts as a potent anti-inflammatory mediator, and has been shown to protect against transplant coronary artery disease and encourage endothelial wound healing (9-11). Although virtually undetectable in the quiescent vasculature, Ang-2 expression is restricted to primarily endothelial cells and dramatically increased at sites that are undergoing vascular remodeling in the setting of endothelial stress. Known stimulants of Ang-2 transcription include environmental factors, most notably hypoxia, and cytokines including vascular endothelial growth factor (VEGF) and TNF (12-14). Once transcribed, Ang-2 is stored in Weibel-Palade Bodies (WPBs) within the endothelial cell, and is thus immediately available for release in the setting of the above stimuli as well as known WPB secretagogues, which include histamine and, importantly for the present study, thrombin (15,16). Once released, Ang-2 rapidly promotes endothelial instability and triggers a potent inflammatory response by priming the endothelium for pro-inflammatory cytokine effects and, as a result, increasing vascular permeability (17,18). The presence of pro-angiogenic cytokines (e.g., VEGF, FGF) in the vascular milieu promotes endothelial cell migration and proliferation, thus stimulating new vessel growth (19). Ang-2 functions antagonistically to Ang-1 at the level of the Tie-2 receptor, by rapidly altering the Ang-1/Tie-2 ratio in favor of vascular stimulation, inflammation, and angiogenesis; the exact mechanism of which likely involves adhesion molecules, and B1-integrin specifically (20). Importantly, it is possible that Ang-2’s effects on Tie-2 are temporally and spatially regulated. For example, it has been shown that sustained Ang-2 stimulation results in activation of the Akt signaling pathway promoting Ang-1-like cell survival, and differential Ang-2 effects have been observed in different vascular beds (19,21). In sum, the authors suggest that increased shear stress from the CF-LVAD as well as direct activation of the contact coagulation cascade leads to increased release of Ang-2 due to direct endothelial injury and by thrombin-mediated WPB release. In the presence of VEGF and other pro-angiogenic factors the vasculature is destabilized, permeability is increased, and new vessels are formed, which are particularly at risk for bleeding in the setting of therapeutic anticoagulation.
Multiple points merit further discussion, particularly prior to suggestion of therapeutic application of Ang-2 blockade. First, it is important to note that the study had a cross sectional design, assessing serum from multiple subjects in each group (CF-LVAD, HF, OHT) at a single time point. Given the significant variability of Ang-2 levels and its elevation in multiple other disease states—including asthma, vasculitis, cancer, and multisystem organ failure; perhaps a more informative approach would have been a longitudinal assessment of Ang-2 levels before and after CF-LVAD implantation, where each subject could serve as their own control, lending insight into the expected variability of Ang-2 levels over time as well as the effects of other disease states within the same individual. It is also important to note that the study did not include disease-free controls, and therefore the effects of Ang-2 in the serum of those patients without HF or transplantation was not addressed. This is particularly important given the extremely high levels of Ang-2 found in all three arms when compared to prior reported levels in healthy controls (22,23). Small sample size within the CF-LVAD group also limited the authors’ ability to carry out powerful subgroup analyses.
While the authors acknowledge the lack of direct evidence that elevated Ang-2 leads to increased AVM formation in vivo, they do demonstrate increased bleeding events in the CF-LVAD group as compared to OHT and HF. In the tables presented, however, no data is shown outlining the details of these bleeding events. While previous studies suggest the majority of NSB in CF-LVAD patients is AVM related, it would be helpful, for example, to know the patient’s INR at the time of the bleeding event and when it occurred during device support (24). Additionally, the finding that Ang-2 levels correlate with events in the first 3 months post-collection with diminishing of this effect at 6 months’ time is unexpected—suggesting either marked variability in Ang-2 levels or possibly significant heterogeneity in what is an already small sample size. Another puzzling result is the marked difference in Ang-2 levels and bleeding events seen in Heartware HVAD patients versus Heartmate II patients, particularly given clinical studies without suggestion of increased bleeding events in HVAD patients (24). It is also interesting that despite Ang-2 levels significantly elevated beyond expected normal levels in all three groups, an increase in NSB was only observed in the CF-LVAD group. Is therapeutic anticoagulation simply uncovering a common problem in all these disease states? Or is there a threshold level for Ang-2 above which the risk of AVM formation or GIB significantly increases?
A clinically relevant question that remains is whether Ang-2 could be used as a biomarker for future bleeding events in CF-LVAD patients. This question can be addressed by a prospective multicenter clinical study where patients are stratified into high and low-risk categories based on Ang-2 levels and followed over time for bleeding events. If Ang-2 remains a significant predictor for future bleeding events, anti-thrombotic therapies can be potentially tailored in patients who are deemed too high-risk for bleeding before these events occur. What is also unclear at the time of this writing is whether or not the favorable effects of Ang-2 blockade in vitro can be successfully translated in vivo. In other words, can Ang-2 blockade in CF-LVAD patients prevent and/or regress formation of AVMs and lead to a reduction in bleeding events? While the current study represents a crucial step toward understanding the mechanisms of AVM formation in CF-LVAD patients, further research is necessary to strengthen the causal link between Ang-2 and AVM formation in the clinical setting and explore potential roles of other angiogenetic factors. As the authors pointed out, there are several Ang-2 blockers that are in clinical trial stages, however success of these approaches is yet to be proven (25). Ongoing research will address the question of whether Ang-2 can serve as a marker and/or mediator of AVM formation in CF-LVAD patients.
Acknowledgements
This study was supported by Lisa and Mark Schwartz and the Program to Reverse Heart Failure at New York Presbyterian Hospital/Columbia University.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Kai Zhu (Department of Cardiac Surgery, Zhongshan Hospital, Fudan University, Shanghai, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lietz K, Long JW, Kfoury AG, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation 2007;116:497-505. [Crossref] [PubMed]
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007;357:885-96. [Crossref] [PubMed]
- Wever-Pinzon O, Selzman CH, Drakos SG, et al. Pulsatility and the risk of nonsurgical bleeding in patients supported with the continuous-flow left ventricular assist device HeartMate II. Circ Heart Fail 2013;6:517-26. [Crossref] [PubMed]
- Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg 2010;90:1263-9; discussion 1269. [Crossref] [PubMed]
- Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 1996;87:1161-9. [Crossref] [PubMed]
- Papapetropoulos A, Fulton D, Mahboubi K, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem 2000;275:9102-5. [Crossref] [PubMed]
- DeBusk LM, Hallahan DE, Lin PC. Akt is a major angiogenic mediator downstream of the Ang1/Tie2 signaling pathway. Exp Cell Res 2004;298:167-77. [Crossref] [PubMed]
- Tsigkos S, Zhou Z, Kotanidou A, et al. Regulation of Ang2 release by PTEN/PI3-kinase/Akt in lung microvascular endothelial cells. J Cell Physiol 2006;207:506-11. [Crossref] [PubMed]
- Jeon BH, Khanday F, Deshpande S, et al. Tie-ing the antiinflammatory effect of angiopoietin-1 to inhibition of NF-kappaB. Circ Res 2003;92:586-8. [Crossref] [PubMed]
- Nykänen AI, Krebs R, Saaristo A, et al. Angiopoietin-1 protects against the development of cardiac allograft arteriosclerosis. Circulation 2003;107:1308-14. [Crossref] [PubMed]
- Cho CH, Sung HK, Kim KT, et al. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc Natl Acad Sci U S A 2006;103:4946-51. [Crossref] [PubMed]
- Oh H, Takagi H, Suzuma K, et al. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem 1999;274:15732-9. [Crossref] [PubMed]
- Hegen A, Koidl S, Weindel K, et al. Expression of angiopoietin-2 in endothelial cells is controlled by positive and negative regulatory promoter elements. Arterioscler Thromb Vasc Biol 2004;24:1803-9. [Crossref] [PubMed]
- Mandriota SJ, Pepper MS. Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res 1998;83:852-9. [Crossref] [PubMed]
- Fiedler U, Scharpfenecker M, Koidl S, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 2004;103:4150-6. [Crossref] [PubMed]
- Huang YQ, Li JJ, Hu L, et al. Thrombin induces increased expression and secretion of angiopoietin-2 from human umbilical vein endothelial cells. Blood 2002;99:1646-50. [Crossref] [PubMed]
- Fiedler U, Reiss Y, Scharpfenecker M, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 2006;12:235-9. [Crossref] [PubMed]
- Roviezzo F, Tsigkos S, Kotanidou A, et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther 2005;314:738-44. [Crossref] [PubMed]
- Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol 2006;27:552-8. [Crossref] [PubMed]
- Hakanpaa L, Sipila T, Leppanen VM, et al. Endothelial destabilization by angiopoietin-2 via integrin β1 activation. Nat Commun 2015;6:5962. [Crossref] [PubMed]
- Kim I, Kim HG, So JN, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3'-Kinase/Akt signal transduction pathway. Circ Res 2000;86:24-9. [Crossref] [PubMed]
- Zhou L, Lan H, Zhou Q, et al. Plasma angiopoietin-2 is persistently elevated after non-small cell lung cancer surgery and stimulates angiogenesis in vitro. Medicine (Baltimore) 2016;95:e4493. [Crossref] [PubMed]
- Wang ZQ, Sun XL, Su HL, et al. Association between serum angiopoietin-2 concentration and clinicopathological parameters in patients with colorectal cancer. Genet Mol Res 2015;14:15547-52. [Crossref] [PubMed]
- Goldstein DJ, Aaronson KD, Tatooles AJ, et al. Gastrointestinal bleeding in recipients of the HeartWare Ventricular Assist System. JACC Heart Fail 2015;3:303-13. [Crossref] [PubMed]
- Mullard A. Phase III setback for lead angiopoietin inhibitor. Nat Rev Drug Discov 2014;13:877.


