Elevated HOTAIR expression associated with cisplatin resistance in non-small cell lung cancer patients
Introduction
Non-small cell lung cancer (NSCLC) accounts for ~85% of lung cancers. Techniques for early diagnosis and the emergence of new-targeted drugs have greatly increased the survival rate of NSCLC patients. However, for late-stage patients and those with drug resistance, the survival rate is still very low (1). Improving treatment outcomes in late-stage cancer patients, especially drug-resistant patients, is a key area of focus in oncology research.
With the rapid development of sequencing technology in recent years, large numbers of long noncoding RNAs (lncRNA) have been identified. lncRNAs are non-protein-coding RNAs with lengths generally above 200 bp. They play an important role in protein regulation by regulating the chromatin state to influence the expression of neighboring genes (2). Increasing research shows that lncRNAs play a role in lung cancer proliferation, invasion, and prognosis (3,4). Tumor stem cells are a source of tumor recurrence and the development of drug resistance. Increasing evidence shows that lncRNA abnormalities are often observed in tumor stem cells, and abnormal lncRNA expression in tumor stem cells plays an important role in tumor formation, development, metastasis, and drug resistance (5,6). HOTAIR is an lncRNA that was initially found to play an important role in breast cancer metastasis (7,8). HOTAIR is highly expressed in many tumors, including lung cancer tumors (9,10). Expression of HOTAIR in lung cancer is correlated with lymph node metastasis, brain metastasis, and poor prognosis (10-12). However, it is currently not clear whether HOTAIR is related to alterations, amplification, deletions, or point mutations in tumor-related genes (9), or whether there is a correlation with drug resistance and if so, by which mechanism.
This study aimed to determine whether HOTAIR is related to drug resistance in NSCLC patients and preliminarily investigate a possible mechanism in order to form a theoretical basis for the discovery of novel targeted therapies.
Methods
Clinical data
In total, 65 patients [36 men, 29 women, 33 patients <52 years of age, 32 patients ≥52 years of age; median age 52 years (range, 38–80 years)] underwent surgery to treat NSCLC at Henan Provincial People’s Hospital between June 2013 and June 2015. Diagnosis was confirmed by pathology, and patients did not undergo radiation or chemotherapy before surgery. Histopathological types were assigned using WHO pathological staging criteria (13). All patients gave their informed consent, and the experiment was approved by the ethics committee at Henan Provincial People’s Hospital. Total RNA was collected from tumor tissues of 30 patients with squamous carcinoma for which cisplatin treatment after surgery was effective (non-drug-resistant patients) and 35 patients with lung adenocarcinoma for which cisplatin treatment after surgery was ineffective (drug-resistant patients). Written informed consent was obtained from all patients. Drug-resistant patients were those that exhibited progressive disease after undergoing 4 weeks of chemotherapy as evaluated by RECIST efficacy criteria, and non-drug-resistant patients were those that exhibited a response after 4 weeks of chemotherapy. After reverse transcription, quantitative PCR was used to measure HOTAIR expression.
A459 NSCLC cells and drug-resistant strains were obtained from the Henan Provincial People’s Hospital Research Center. Trizol reagent was purchased from Invitrogen (Carlsbad, CA, USA). Reverse transcription reagent kits and fluorescent quantitative PCR reagent kits were purchased from Takara (Dalian, Liaoning, China). Primers were synthesized by Shanghai Invitrogen Biotechnology Co. (Shanghai China). Cisplatin was purchased from Qilu Pharmacy Ltd. Co. (Jinan, Shandong, China). Thiazolyl blue (MTT), epidermal growth factor (EGF), and basic fibroblast growth factor (bFGF) were purchased from Sigma (St. Louis, MO, USA). B27 was purchased from Shanghai Invitrogen Co. Protein lysis buffer was purchased from Cell Signal Co. (St. Louis, MO, USA). A BCA protein content measurement kit was purchased from CWBIO (Shanghai, China). Nanog, Oct3/4, Sox2, c-Myc, β-catenin, GAPDH antibodies (diluted 1:100) were purchased from Santa Cruz Biotechnology (Dallas, TX, U.S.A). Klf4 was purchased from Abcam (Cambridge, Cambridgeshire, England). Cell culture media, antibiotics and antimycotics, and fetal bovine serum were purchased from Gibco (New York, NY, USA).
RNA isolation
Cryopreserved NSCLC tissue was crushed into a powder and lysed for 10 min by addition of 1 mL of Trizol reagent. The supernatant was transferred to an Eppendorf (EP) tube and 200 µL of chloroform was added and mixed. The mixture was centrifuged at 12,000 rpm for 15 min, and 200 µL of the supernatant was transferred to a new RNase-free EP tube. An equal volume of isopropanol was added and mixed by inversion. The mixture was centrifuged at 12,000 rpm for 10 min, the supernatant was discarded, and 1 mL of 70% ethanol was added and mixed by inversion. The mixture was centrifuged at 12,000 rpm for 10 min, ethanol was discarded, and the pellet was air dried and resuspended in DEPC-treated distilled water. A Malcom e-spect visible light spectrophotometer (Malcom, Tokyo, Japan) was used to measure the concentration of the isolated RNA; samples with OD260/280 values between 1.8 and 2.0 were used for experiments. Reverse transcription and quantitative PCR were performed following the manufacturer’s instructions.
Lentivirus production and transduction
HOTAIR was cloned into the overexpression vector pCDH-MSCV-mcs-EF1-GFP-T2A-Pu (SBI) after amplification. The pLKO.1 vector was used to connect the knocked-down HOTAIR sequence (5'–CCGGGAACGGGAGTACAGAGAGAATCTCGAGATTCTCTCTGTACTCCCGTTCTTTTTG–3'). The lentiviral packaging plasmids psPAX2 and pMD2.G were kindly provided by Prof. Didier Trono (University of Geneva, Geneva, Switzerland).
Detection of cell drug resistance
Cells were cultured in standard conditions for 48 h. In the absence of light, 20 µL of previously prepared MTT solution (5 mg/mL) was added to each well. Cells were incubated for 4 h, after which the culture medium was discarded and 150 µL of DMSO was added to each well and the plate was gently agitated for 10 min at room temperature. Optical density (OD) was measured at an absorbance wavelength of 490 nm using a microplate reader. The formula for calculating the cell survival rate was: cell survival rate = (OD value of drug-treated group − OD value of empty control group)/(OD value of normal cell control group − OD value of empty control group) ×100%. Half-maximal inhibitory concentration (IC50) calculation software was used to calculate the IC50 of sulforaphane (SF) on the two strains of cells.
Tumorsphere formation
As tumor stem cells, but not tumor cells, can grow in suspension in a serum-free medium, a tumorsphere culture can be used to isolate and characterize stem cells. Cells in the logarithmic growth phase were digested with trypsin, collected by centrifugation at 1,000 rpm for 2 min, and the supernatant was removed. Cells were washed with Hanks’ balanced salt solution three times, and suspended in a tumorsphere serum-free medium containing DMEM/F12 (1:1). B27 supplement (1:50), EGF (25 ng/L), and fibroblast growth factor (25 ng/L) were added. After determination of the cell number and adjusting the density to 1,500 cells/mL, a single cell suspension was evenly transferred into an ultra-low-attachment 6-well plate for the primary tumorsphere culture. After 3–4 days, the number of tumorspheres was counted in three fields at a low magnification; the counting was repeated three times. Tumorsphere diameter was also recorded. Cells of each group in the logarithmic growth phase were collected, trypsinized, centrifuged, and washed 3 times in sterile PBS to completely remove serum. Next, cells were resuspended in previously prepared tumor sphere culture medium as a single-cell suspension. Finally, 1×105 cells were placed in a low-adhesion culture plate and cultured at a constant temperature of 37 °C, 95% humidity, and 5% CO2. For the first 3 days, a 1 mL pipette was used to gently pipette the culture medium up and down every 2 hours in the morning to prevent cell adhesion, and in the evening, cells were collected in a sterile glass centrifuge tube overnight. Each day, 0.25 mL of fresh tumor sphere culture medium was added. On day 14, the number and size of tumor spheres formed was determined for cells of each group, and statistical analysis was performed.
Western blot
A 10% resolving gel and a 5% stacking gel were used for immunoblotting experiments. After loading prepared samples in the gel, electrophoresis was performed through the stacking gel at 80 V for 30 min and through the resolving gel at 100 V for 90 min. The gel was transferred to a membrane using a low-temperature constant current of 350 mA for 120 min. The membrane was sealed with BSA for 1 h, incubated with a primary antibody overnight at 4 °C, and developed and imaged using a gel documentation system (BIO-RAD, Hercules, CA, USA). The membrane was incubated with a secondary antibody for 2 h at room temperature, and the results were analyzed.
Statistics
SPSS (v13.0) software (Chicago, IL, USA) was used to perform statistical analysis. Data were reported as mean ± standard deviation. Two-sample means were compared using a Student’s t tests, multiple-sample means were compared using complete randomized block one-way ANOVA, and pairwise multiple-sample means were compared using LSD and Bonferroni tests. P<0.05 was considered statistically significant, and P<0.01 was considered very statistically significant.
Results
There was no significant correlation between the occurrence of cisplatin resistance and clinical parameters (Table 1). HOTAIR expression did not significantly correlate with sex, age, pathological type, T classification or clinical stage, but significantly correlated with pathological grade (P<0.01, Table 1).
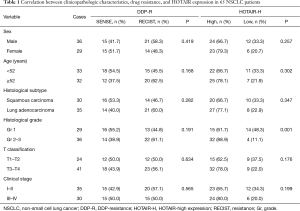
Full table
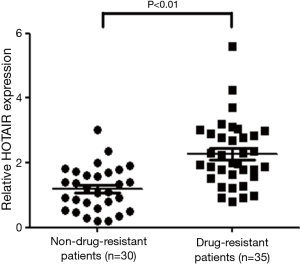
Elevated expression of HOTAIR in drug-resistant NSCLC patients
Comparing HOTAIR expression in the tissues of drug-resistant and non-drug-resistant patients, HOTAIR expression in the cisplatin-resistant group was greater than that in the non-resistant group (P<0.01; Figure 1). This result indicates that elevated HOTAIR expression was associated with cisplatin resistance in NSCLC patients.
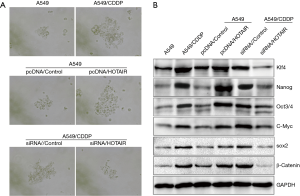
Elevated HOTAIR expression promotes cisplatin resistance
Our observations of elevated HOTAIR expression in drug-resistant patient tissue specimens prompted further investigation of this factor. We selected the A549 cell line and its cisplatin-resistant strain (A549/CDDP) to conduct additional experiments. We found that HOTAIR was highly expressed in A549/CDDP cells (Figure 2A). Next, we upregulated HOTAIR expression in A549 cells and knocked down HOTAIR expression in A549/CDDP cells (Figures 2B,C). Using cell survival experiments, we observed that overexpression of HOTAIR in A549 cells promoted the development of cisplatin resistance, whereas the upregulation and downregulation of HOTAIR expression in A549/CDDP cells promoted cisplatin sensitivity (Figure 2D). This result indicated that elevated HOTAIR expression was implicated in cisplatin resistance.

Drug resistance induced by elevated HOTAIR expression may be caused by promotion of tumor sphere cell growth and induction of tumor stem cell biomarker expression
Tumor stem cells are one of the primary factors in the development of drug resistance. Elevated expression of HOTAIR affects the biology of tumor stem cell colonies, which in turn influences drug resistance. We performed tumorsphere formation experiments using A549 cells, A549/CDDP cells, A549 cells overexpressing HOTAIR, and A549/CDDP cells with HOTAIR knocked-down. We found that increased HOTAIR expression promoted tumor sphere formation, whereas reduced HOTAIR expression decreased tumor sphere formation (Figure 3A). Western blot experiments showed that elevated HOTAIR expression upregulated expression of the tumor stem cell-related biomarkers Nanog, Oct3/4, Sox2, c-Myc, β-catenin, and Klf4 (Figure 3B). These results indicate that elevated HOTAIR expression can induce upregulation of tumor stem cell-related biomarkers, which could be a molecular mechanism by which cells develop drug resistance.
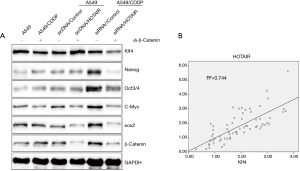
Elevated HOTAIR expression upregulates Klf4 expression in β-catenin-deficient cells; HOTAIR expression and Klf4 expression are positively correlated
HOTAIR expression influences expression of Nanog, Oct3/4, Sox2, c-Myc, β-catenin, and Klf4. Because the gene encoding β-catenin is upstream of those encoding Nanog, Oct3/4, Sox2, c-Myc, and Klf4, we knocked down β-catenin in the context of elevated HOTAIR expression. Western blot experiments showed that expression of Nanog, Oct3/4, Sox2, and c-Myc was not affected by HOTAIR expression, whereas that of Klf4 was still affected (Figure 4A). Thus, HOTAIR regulation of Klf4 expression was not regulated by β-catenin. Next, we analyzed the correlation between Klf4 and HOTAIR, and found that the two genes positively correlated, with a Pearson coefficient of R2=0.744 (Figure 4B).
Discussion
Cisplatin is a first-line chemotherapy drug against NSCLC, but cisplatin resistance among NSCLC patients has become increasingly severe and represents an important cause of chemotherapy failure (14,15). lncRNAs are a major research focus, and their number exceeds that of protein-coding RNAs. An increasing number of lncRNAs that participate in gene regulation are being discovered (16). Ever since the discovery of HOTAIR, an important representative lncRNA, research has shown that it is abnormally expressed in many types of tumors. Elevated HOTAIR expression is an important factor that promotes breast cancer metastasis and poor prognosis, and regulates polycomb repressive complex 2 (PRC2) by regulating target genes, thereby participating in gene expression by changing the methylation of histone H3 lysine 27 and promoting tumor invasion and metastasis (8,17). In lung adenocarcinoma, increased HOTAIR expression inhibits p21, thereby promoting proliferation and cisplatin resistance (10). Our results show that HOTAIR expression is abnormal in NSCLC; specifically, in the tissues of cisplatin-resistant NSCLC patients, its expression level was increased compared with that of the non-drug-resistant group.
Research has shown that cisplatin resistance in NSCLC is closely related to lncRNA status and abnormal expression of HOX loci was detected in tissue specimens from patients with advanced NSCLC (15). Our research showed that HOTAIR was expressed in A549 and A549/CDDP cells and that its expression levels were higher in drug-resistant strains than in non-drug-resistant strains. HOTAIR overexpression in A549 cells promoted cisplatin resistance, and knocked down HOTAIR in A549/CDDP cells reduced cisplatin resistance.
The cause of drug resistance in tumors is heterogeneous, as cellular components in tumor tissues have different sensitivities to chemotherapy. Compared with normal tumor cells, tumor stem cells are not easily killed by chemotherapy drugs (18). Tumor stem cells are a small population of special cells among other tumor cells with the potential to self-renew and differentiate, and are an important reason for tumor invasion, metastasis, and drug resistance (19-21). Tumor stem cells have a strong tendency to form spheres in vitro, and a minimal number of tumor stem cells can form tumors subcutaneously in nude mice; in addition, tumor stem cells are often drug-resistant (22). Studies have shown that lncRNAs also play an important role in tumor stem cells (23). HOTAIR is a commonly observed lncRNA and can promote changes in tumor formation, metastasis and degree of differentiation by affecting the stemness of tumor stem cells (24-27). Our in vitro sphere formation assay showed that the ability of A549/CDDP cells to form spheres in vitro was greater than that of A549 cells. Further experiments showed that upregulation of HOTAIR expression in A549 cells resulted in an increased volume in tumor spheres, whereas knocked down HOTAIR in A549/CDDP cells inhibited tumor sphere growth. These results show that elevated HOTAIR expression in tumor stem cells promotes stemness. Further, western blot experiments showed that HOTAIR promoted expression of β-catenin, Nanog, Oct3/4, Sox2, c-Myc, and Klf4. These are important biomarkers of tumor stem cells, and are highly expressed in many tumors and strongly associated with tumor invasion, metastasis, and poor prognosis (28). Elevated expression of HOTAIR promoted expression of these biomarkers of stemness, which could lead to the development of drug resistance.
With respect to understanding the mechanism by which HOTAIR potentially functions in lung cancer, the greatest obstacle is that there is no standard method for predicting downstream sites of action (29,30). We know that the β-catenin gene is upstream of Nanog, Oct3/4, Sox2, c-Myc, and Klf4, and our previous studies have shown that there is a correlation between β-catenin and Nanog in lung cancer (31,32). Moreover, elevated β-catenin expression can promote Nanog, Oct3/4, Sox2, c-Myc, and Klf4 expression (33,34). To determine how HOTAIR promotes β-catenin, Nanog, Oct3/4, Sox2, c-Myc, and Klf4 expression, we knocked down β-catenin expression in the context of elevated HOTAIR expression, and found that Nanog, Oct3/4, Sox2, and c-Myc expression was no longer regulated by HOTAIR but Klf4 was still regulated by HOTAIR. These results led us to believe that HOTAIR regulation of Klf4 is at least partially independent of β-catenin. Further clinical tissue specimen analysis showed that HOTAIR expression and Klf4 expression positively correlated, further demonstrating that HOTAIR may directly regulate Klf4 expression. Furthermore, we also observed that although there was a statistically significant difference, individual patients had the opposite result for Klf4 and HOTAIR expression. This result could be related to Klf4 function, which is different depending on subcellular localization (cell membrane, cytoplasm, or nucleus) (35,36). Thus, the specific functions and interactions of HOTAIR in the cell membrane, cytoplasm, and nucleus require further investigation.
Elevated HOTAIR expression in NSCLC is an important factor in the development of cisplatin resistance, and HOTAIR may directly regulate Klf expression to promote stemness. The discovery of potential therapies targeting HOTAIR provides a novel molecular strategy for treatment of cisplatin-resistant NSCLC patients.
Acknowledgements
Funding: The work was funded by the Natural Science Foundation of China (No. U1504820).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The experiment was approved by the ethics committee at Henan Provincial People’s Hospital (approval number: ZD5020165294). All patients gave their informed consent.
References
- Lopez-Ayllon BD, Moncho-Amor V, Abarrategi A, et al. Cancer stem cells and cisplatin-resistant cells isolated from non-small-lung cancer cell lines constitute related cell populations. Cancer Med 2014;3:1099-111. [Crossref] [PubMed]
- Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell 2011;43:904-14. [Crossref] [PubMed]
- Luo J, Tang L, Zhang J, et al. Long non-coding RNA CARLo-5 is a negative prognostic factor and exhibits tumor pro-oncogenic activity in non-small cell lung cancer. Tumour Biol 2014;35:11541-9. [Crossref] [PubMed]
- Qiu M, Xu Y, Yang X, et al. CCAT2 is a lung adenocarcinoma-specific long non-coding RNA and promotes invasion of non-small cell lung cancer. Tumour Biol 2014;35:5375-80. [Crossref] [PubMed]
- Huang T, Alvarez A, Hu B, et al. Noncoding RNAs in cancer and cancer stem cells. Chin J Cancer 2013;32:582-93. [Crossref] [PubMed]
- Deng G, Sui G. Noncoding RNA in oncogenesis: a new era of identifying key players. Int J Mol Sci 2013;14:18319-49. [Crossref] [PubMed]
- Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010;464:1071-6. [Crossref] [PubMed]
- Grigorescu AC. Chemotherapy for elderly patients with advanced cancer: A pilot study in Institute of Oncology Bucharest. J Transl Int Med 2015;3:24-8. [Crossref] [PubMed]
- Loewen G, Jayawickramarajah J, Zhuo Y, et al. Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol 2014;7:90. [Crossref] [PubMed]
- Zhao W, An Y, Liang Y, et al. Role of HOTAIR long noncoding RNA in metastatic progression of lung cancer. Eur Rev Med Pharmacol Sci 2014;18:1930-6. [PubMed]
- Nakagawa T, Endo H, Yokoyama M, et al. Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease-free survival in human non-small cell lung cancer. Biochem Biophys Res Commun 2013;436:319-24. [Crossref] [PubMed]
- Liu XH, Liu ZL, Sun M, et al. The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer 2013;13:464. [Crossref] [PubMed]
- Petersen I, Warth A. Lung cancer: developments, concepts, and specific aspects of the new WHO classification. J Cancer Res Clin Oncol 2016;142:895-904. [Crossref] [PubMed]
- Galluzzi L, Vitale I, Michels J, et al. Systems biology of cisplatin resistance: past, present and future. Cell Death Dis 2014;5:e1257. [Crossref] [PubMed]
- Hou Z, Xu C, Xie H, et al. Long noncoding RNAs expression patterns associated with chemo response to cisplatin based chemotherapy in lung squamous cell carcinoma patients. PLoS One 2014;9:e108133. [Crossref] [PubMed]
- Zeng S, Xiao YF, Tang B, et al. Long Noncoding RNA in Digestive Tract Cancers: Function, Mechanism, and Potential Biomarker. Oncologist 2015;20:898-906. [Crossref] [PubMed]
- Zhang J, Zhang P, Wang L, et al. Long non-coding RNA HOTAIR in carcinogenesis and metastasis. Acta Biochim Biophys Sin (Shanghai) 2014;46:1-5. [Crossref] [PubMed]
- Alison MR, Lim SM, Nicholson LJ. Cancer stem cells: problems for therapy? J Pathol 2011;223:147-61. [Crossref] [PubMed]
- Huang Z, Wu T, Liu AY, et al. Differentiation and transdifferentiation potentials of cancer stem cells. Oncotarget 2015;6:39550-63. [PubMed]
- Farnie G, Sotgia F, Lisanti MP. High mitochondrial mass identifies a sub-population of stem-like cancer cells that are chemo-resistant. Oncotarget 2015;6:30472-86. [PubMed]
- Teschendorff AE, Lee SH, Jones A, et al. HOTAIR and its surrogate DNA methylation signature indicate carboplatin resistance in ovarian cancer. Genome Med 2015;7:108. [Crossref] [PubMed]
- Kryczek I, Liu S, Roh M, et al. Expression of aldehyde dehydrogenase and CD133 defines ovarian cancer stem cells. Int J Cancer 2012;130:29-39. [Crossref] [PubMed]
- Yang Q, Zhang RW, Sui PC, et al. Dysregulation of non-coding RNAs in gastric cancer. World J Gastroenterol 2015;21:10956-81. [Crossref] [PubMed]
- Endo H, Shiroki T, Nakagawa T, et al. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One 2013;8:e77070. [Crossref] [PubMed]
- Li H, An J, Wu M, et al. LncRNA HOTAIR promotes human liver cancer stem cell malignant growth through downregulation of SETD2. Oncotarget 2015;6:27847-64. [Crossref] [PubMed]
- Wang J, Chen D, He X, et al. Downregulated lincRNA HOTAIR expression in ovarian cancer stem cells decreases its tumorgeniesis and metastasis by inhibiting epithelial-mesenchymal transition. Cancer Cell Int 2015;15:24. [Crossref] [PubMed]
- Grouse L. Translational Genetic Research of Complex Diseases. J Transl Int Med 2015;3:137-43. [PubMed]
- Nishiguchi M, Kikuyama H, Kanazawa T, et al. Increases in iPS Transcription Factor (Oct4, Sox2, c-Myc, and Klf4) Gene Expression after Modified Electroconvulsive Therapy. Psychiatry Investig 2015;12:532-7. [Crossref] [PubMed]
- Kirmizis A, Bartley SM, Kuzmichev A, et al. Silencing of human polycomb target genes is associated with methylation of histone H3 Lys 27. Genes Dev 2004;18:1592-605. [Crossref] [PubMed]
- Cheng LL, Liu Y, Su Z, et al. Clinical characteristics of tobacco smoke-induced versus biomass fuelinduced chronic obstructive pulmonary disease. J Transl Int Med 2015;3:126-9. [PubMed]
- Li XQ, Yang XL, Zhang G, et al. Nuclear β-catenin accumulation is associated with increased expression of Nanog protein and predicts poor prognosis of non-small cell lung cancer. J Transl Med 2013;11:114. [Crossref] [PubMed]
- Marucci L, Pedone E, Di Vicino U, et al. β-catenin fluctuates in mouse ESCs and is essential for Nanog-mediated reprogramming of somatic cells to pluripotency. Cell Rep 2014;8:1686-96. [Crossref] [PubMed]
- Li W, Tian E, Chen ZX, et al. Identification of Oct4-activating compounds that enhance reprogramming efficiency. Proc Natl Acad Sci U S A 2012;109:20853-8. [Crossref] [PubMed]
- Madeja ZE, Hryniewicz K, Orsztynowicz M, et al. WNT/β-catenin signaling affects cell lineage and pluripotency-specific gene expression in bovine blastocysts: prospects for bovine embryonic stem cell derivation. Stem Cells Dev 2015;24:2437-54. [Crossref] [PubMed]
- Pandya AY, Talley LI, Frost AR, et al. Nuclear localization of KLF4 is associated with an aggressive phenotype in early-stage breast cancer. Clin Cancer Res 2004;10:2709-19. [Crossref] [PubMed]
- Chen YJ, Wu CY, Chang CC, et al. Nuclear Krüppel-like factor 4 expression is associated with human skin squamous cell carcinoma progression and metastasis. Cancer Biol Ther 2008;7:777-82. [Crossref] [PubMed]


