Elevated high-sensitivity C-reactive protein in preterm infants with pulmonary colonization with Ureaplasma
Introduction
Originally described by Northway and Rosan in 1967, bronchopulmonary dysplasia (BPD) is a chronic lung disease of prematurity (1). Despite advances in medical management of very low birth weight (VLBW) infants, BPD is still observed in 30% of infants less than 28 weeks gestation (2) and poses a significant burden of disease. Affected infants are at risk for extended hospitalization length, prolonged exposure to oxygen, and prolonged mechanical ventilation time. Patients with BPD who are discharged from the hospital are at increased risk for readmission, recurrent respiratory infections, airway reactivity, and higher mortality. Older definitions of BPD included pathophysiologic mechanisms involving significant inflammation, pulmonary edema, and alveolar destruction. As surfactant therapy was introduced and widely utilized, the pathology of BPD has changed rendering the “new” BPD to be defined as a persistent oxygen requirement or need for respiratory support at 36 weeks postmenstrual age (BPD36). Along with the clinical evolution of BPD, the pathological presentation likewise evolved. BPD36 is associated with more uniform lung inflation, fewer but larger alveoli, less fibrosis, and less fulminant but persistent inflammation (2).
The association of Ureaplasma spp. presence in premature infant lungs with BPD development and/or inflammatory responses remains unclear and controversial. Many studies suggest Ureaplasma spp. are associated with BPD and pulmonary inflammation (2-11), while others dispute their significance (5,12). However, most research does support the link between Ureaplasma spp. colonization and pulmonary inflammation with eventual development of BPD. Proinflammatory cytokines have been detected in the tracheal aspirates of infants with Ureaplasma spp. colonization, suggesting a link to pulmonary inflammation (13-15). Further, Panero et al. detected an increased systemic inflammatory response in neonates with clinical and laboratory signs of Ureaplasma pneumonia (9). This finding suggests that inflammation may not be localized to the pulmonary system, but may be more widespread and lead to a systemic inflammatory response. With the advent of C-reactive protein (CRP), there is a detectable systemic inflammatory marker available with the ability to detect even lower levels using high sensitivity CRP (hs-CRP) (13-17). Our goal was to identify if a systemic inflammatory marker is present in VLBW premature infants colonized with Ureaplasma spp. using serum hs-CRP as a biomarker. Therefore, our hypothesis was that a systemic inflammatory response as measured by hs-CRP is present in VLBW premature neonates colonized with Ureaplasma spp. as compared to non-colonized controls.
Methods
Study design and setting
We completed a prospective cohort study that was approved by the University of Kentucky Institutional Review Board. Subjects enrolled were VLBW premature infants in the neonatal intensive care unit between July 2007 and July 2008. Informed consent for participation in this project was obtained for each subject enrolled. Eligibility for enrollment included premature infants less than 28 weeks gestation, birth weight of 1,250 g or less, and endotracheal intubation within the first 72 hours of life. Exclusion criterion included any congenital anomalies, congenital heart disease, suspected or confirmed maternal chorioamnionitis, early onset neonatal sepsis, inability to collect both early and late hs-CRP values, or lack of informed consent. Data collected from the medical record for each patient enrolled included birth weight, gestational age, and severity of BPD. Both serum and tracheal aspirate samples were collected during the first 28 days of life, representing the early hs-CRP group, while repeat samples were obtained between 28-42 days of life for the late hs-CRP group. Ureaplasma positivity was defined as positive polymerase chain reaction (PCR) at any time during the study period.
Blood samples
Approximately one mL of blood was collected during initial intravenous or umbilical cannulation and at routine morning blood collection times. After collection, samples were transported on ice and immediately centrifuged at 1,500 g for 30 seconds at room temperature to separate serum from solid elements. Serum was collected and stored at –80 °C and batch analyzed every six months. Batch analysis of hs-CRP was performed using the hs-CRP Enzyme Immunoassay Test Kit (MP Biomedicals; Orangeburg, NY Catalog Number 07BC-1119).
Every six months each sample was removed from -80° storage, slow thawed on ice and allowed to come to room temperature. Prior to analysis each sample was again centrifuged at 1,500 g for 30 seconds. Each sample was then analyzed according to the procedures outlined in the hs-CRP test kit. All standards and patient samples were analyzed in triplicate. Absorbances of each sample were analyzed using a Molecular Devices Spectramax plus 384 microwell reader capable of recording absorbance at 450 nm. Softmax Pro 4.6 was used to plot standard curves and calculate hs-CRP results per the instructions provided in the immunoassay test kit.
Tracheal aspirates
Tracheal aspirates were collected for analysis by polymerase chain reaction (PCR). Aspirates were collected at initial intubation, third day of life and weekly thereafter. Fluid was collected from the endotracheal tube into a sterile trap via suctioning after instillation of sterile saline (0.9%), 0.5 mL in two aliquots. Tracheal aspirates were transported on ice and immediately processed by centrifugation at 1,000 g for 10 minutes to allow separation of the supernatant and cell pellet. Both were then frozen at –80 °C. Batch analysis of PCR was later performed. Every six months samples were slow thawed on ice and re-centrifuged at 300 g for 5 minutes. The cell pellet was collected and resuspended in 200 µL of 1× PBS (pH 7.4 KCl 2.7 mM, Potassium phosphate 1.8 mM, NaCl 137 mM, Sodium phosphate 10.1 mM). DNA from the samples was then extracted using the Qiagen DNeasy Kit (Cat. No. 69506).
Briefly, each sample was mixed with 20 µL of Proteinase K buffer [TRIS HCL 7.5 10 mM, CaCl2 20 mM, Glycerol 50%] and 200 µL of buffer AL (proprietary mixture from Qiagen) and incubated for 10 minutes in a 70 degree Celsius water bath. PCR was performed to detect the presence of Ureaplasma spp. using the U5 sense (5-CAATCTGCTCGTGAAGTATTAC-3) and U4 antisense (5-ACGACGTCCATAAGCAACT-3) primers specific for the urease structural gene. Each PCR batch included two positives (U. parvum, U. urealyticum, ATCC, Manassas, VA) and one negative control (M. hominis, ATCC, Manassas, VA). Samples underwent 35 cycles of denaturation at 95 °C for 30 seconds in a thermal cycler, thirty seconds of primer annealing at 62 °C, and extension for one minute at 72 °C. Amplified products were analyzed by electrophoresis and DNA fragments were visualized by ultraviolet fluorescence.
Statistical analysis
Data analyzed for each patient enrolled included birth weight, gestational age, sex, severity of BPD, colonization with Ureaplasma urealyticum, and hs-CRP level. Statistical analysis to analyze differences between Ureaplasma spp. colonized and non-colonized infants was performed using Mann Whitney test. Due to data not being normally distributed, the data are reported as median with interquartile range. P values of <0.05 were considered statistically significant.
Results
Sixty-five patients were enrolled with a total of 30 infants meeting final inclusion criteria for full analysis in the study. Fifteen patients died before completion of the study, while twenty patients did not have both early and late hs-CRP value measured to compare and are included only in partial analysis. Thus, 30/65 subjects enrolled in this study had a complete data set of early and late hs-CRP as well as PCR analysis for Ureaplasma spp. 40% (12/30) of infants had Ureaplasma spp. identified on tracheal aspirate and 60% (18/30) not. Table 1 outlines demographic data (gestational age, birth weight, and antenatal corticosteroid administration) among patient groups. An equal number of males and females are represented. Racial demographics included 26 Caucasians and four Black infants. All patients received surfactant therapy, but only seven received prophylactic surfactant treatment in the delivery room. The majority of infants received at least a partial course of antenatal corticosteroids and was less than 26 weeks gestational age with a birth weight of equal to or less than 1,200 g.
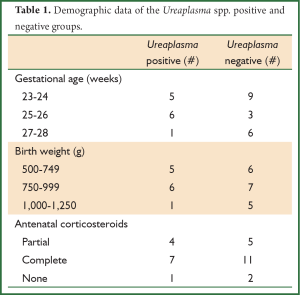
Full Table
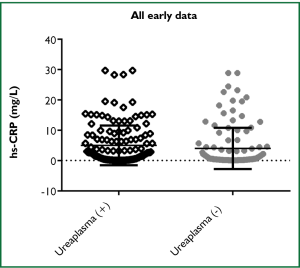
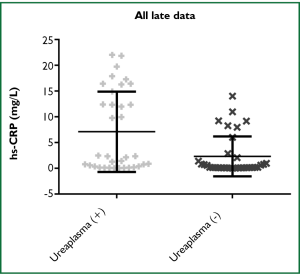
Comparing all data for both early hs-CRP and late hs-CRP levels (mg/L), there was no significant difference between the two groups, the median was 1.019 [0.242, 5.844] vs. 0.773 [0.143, 8.954] (P=0.3958). However, there was a significant difference when comparing Ureaplasma spp. colonization vs. non-colonization between the individual groups. For the early hs-CRP group, the median was 2.223 [0.398, 7.099] vs. 0.675 [0.219, 4.038] (P=0.0131) (Figure 1). For the late hs-CRP group, the median was 2.335 [0.359, 14.91] vs. 0.2155 [0.122, 2.296] (P=0.03) (Figure 2). These data remained significant when cleansed of outliers (not shown).
Discussion
CRP synthesis is primarily regulated by interleukin 6 in response to an inflammatory stimulus. Originally, assays for CRP detection were limited to a lower level of detection of 3-5 mg/L. The advent of hs-CRP use permits detection of lower levels of serum CRP allowing for higher sensitivity detection of inflammation. High sensitivity testing has been used to predict cardiovascular outcomes, endocrine disorders, cancer mortality, and inflammatory asthma phenotypes (18-21). The role of hs-CRP in premature infants is less well established. In newborns, IL-6 and CRP are known to rise within hours after birth even in term infants, and then quickly return to normal levels (22). Likewise, VLBW infants often experience adverse perinatal events and require aggressive resuscitation measures which could predispose them to an early inflammatory response. We attempted to minimize such confounders by excluding infants born to mothers with documented chorioamnionitis and those infants with culture proven bacterial sepsis at the time of enrollment.
Our findings lend further support to a systemic inflammatory response associated with Ureaplasma spp. in premature neonates. Ambalavanan et al. described an association of elevated CRP on day of life 28 in extremely low birth weight infants with BPD36 and/or death even with exclusion of sepsis (23). Furthermore, Viscardi et al. reported that the presence of systemic inflammation as measured by elevations in peripheral white blood cell and neutrophil counts in Ureaplasma infected infants (6). Compared to most previous studies, the primary limitations of our study are the relatively low number of enrolled patients, the lack of a control group who did not develop BPD36, and missing data from dead or transferred patients. We were also unable to evaluate the association of hs-CRP with incidence of BPD because every patient enrolled in this study ultimately developed BPD. Likewise, no conclusions could be made about systemic inflammatory markers and severity of BPD because the majority of enrolled patients (85%) developed moderate to severe BPD.
Based on these findings, the presence of Ureaplasma in tracheal aspirates of VLBW premature neonates resulted in an elevation of early and late hs-CRP, indicative of a chronic systemic inflammatory response. Due to study limitations, we cannot address the contribution of this inflammatory response to the development of BPD. We demonstrated higher serum levels hs-CRP initially that persisted for 42 days in infants colonized with Ureaplasma spp. as compared to non-colonized patients. These findings correlate well with the evolving definition of BPD36. After the introduction of pulmonary surfactant and lower tidal volume ventilation techniques the histopathology of BPD has changed from significant early pulmonary inflammation to a more chronic, low level inflammation. We demonstrated that a persistent low-grade systemic inflammatory response develops and persists in infants with pulmonary Ureaplasma spp. colonization, which can be easily measured with hs-CRP assay.
Ureaplasma spp. has received much attention in the recent literature as a potential nidus for pulmonary inflammation and eventual development of BPD. Intrauterine bacterial loads of Ureaplasma spp. have been correlated with increased inflammatory markers in amniotic fluid and fetal cord blood as well (24). The debate over the significance of Ureaplasma spp. colonization and inflammatory lung disease in VLBW infants is far from over. Likewise, the documentation of systemic inflammation due to such pulmonary pathology is yet to be determined. In conclusion, we discovered a persistent elevation in hs-CRP in VLBW premature infants with Ureaplasma spp. pulmonary colonization when compared to non-colonized infants, suggesting a chronic low-grade systemic inflammatory response in this patient population. Further study is needed to identify if a link exists between hs-CRP and severity of BPD36, pulmonary Ureaplasma spp. bacterial load, or other risk factors of lung disease. Such a link could provide clinicians with a rapid, relatively cost effective biomarker to signify the need for intervention in infants with associated risk factors for severe lung disease.
Acknowledgements
The work and subsequent manuscript was completed at the University of Kentucky, Lexington, KY. The project was completed using internal funding provided by the University of Kentucky Department of Pediatrics.
Disclosure: The authors declare no conflict of interest.
References
- Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967;276:357-68. [PubMed]
- Viscardi RM, Hasday JD. Role of Ureaplasma species in neonatal chronic lung disease: epidemiologic and experimental evidence. Pediatr Res 2009;65:84R-90R. [PubMed]
- Agasthian T, Deschamps C, Trastek VF, et al. Surgical management of bronchiectasis. Ann Thorac Surg 1996;62:976-8; discussion 979-80. [PubMed]
- Kotecha S, Hodge R, Schaber JA, et al. Pulmonary Ureaplasma urealyticum is associated with the development of acute lung inflammation and chronic lung disease in preterm infants. Pediatr Res 2004;55:61-8. [PubMed]
- Seaton D. Bronchiectasis. In: Seaton A, Seaton D. eds. Crofton and Douglas’s respiratory diseases. Volume I. 5th ed. New Delhi: Oxford University Press, 2000:794-808. [PubMed]
- Prieto D, Bernardo J, Matos MJ, et al. Surgery for bronchiectasis. Eur J Cardiothorac Surg 2001;20:19-23, discussion 23-4. [PubMed]
- Groneck P, Schmale J, Soditt V, et al. Bronchoalveolar inflammation following airway infection in preterm infants with chronic lung disease. Pediatr Pulmonol 2001;31:331-8. [PubMed]
- Ambalavanan N, Carlo WA, D’Angio CT, et al. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics 2009;123:1132-41. [PubMed]
- Panero A, Pacifico L, Rossi N, et al. Ureaplasma urealyticum as a cause of pneumonia in preterm infants: analysis of the white cell response. Arch Dis Child Fetal Neonatal Ed 1995;73:F37-40. [PubMed]
- Colaizy TT, Morris CD, Lapidus J, et al. Detection of ureaplasma DNA in endotracheal samples is associated with bronchopulmonary dysplasia after adjustment for multiple risk factors. Pediatr Res 2007;61:578-83. [PubMed]
- Schelonka RL, Waites KB. Ureaplasma infection and neonatal lung disease. Semin Perinatol 2007;31:2-9. [PubMed]
- Aaltonen R, Vahlberg T, Lehtonen L, et al. Ureaplasma urealyticum: no independent role in the pathogenesis of bronchopulmonary dysplasia. Acta Obstet Gynecol Scand 2006;85:1354-9. [PubMed]
- Tsimikas S, Willerson JT, Ridker PM. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J Am Coll Cardiol 2006;47:C19-31. [PubMed]
- Pinto-Plata VM, Müllerova H, Toso JF, et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax 2006;61:23-8. [PubMed]
- Clearfield MB. C-reactive protein: a new risk assessment tool for cardiovascular disease. J Am Osteopath Assoc 2005;105:409-16. [PubMed]
- de Ferranti SD, Rifai N. C-reactive protein: a nontraditional serum marker of cardiovascular risk. Cardiovasc Pathol 2007;16:14-21. [PubMed]
- Takemura M, Matsumoto H, Niimi A, et al. High sensitivity C-reactive protein in asthma. Eur Respir J 2006;27:908-12. [PubMed]
- Ko YJ, Kwon YM, Kim KH, et al. High-sensitivity C-reactive protein levels and cancer mortality. Cancer Epidemiol Biomarkers Prev 2012;21:2076-86. [PubMed]
- Kitsios K, Papadopoulou M, Kosta K, et al. High-Sensitivity C-Reactive Protein Levels andMetabolic Disorders in Obese and OverweightChildren and Adolescents. J Clin Res Pediatr Endocrinol 2013;5:44-9. [PubMed]
- Raposeiras Roubín S, Barreiro Pardal C, et al. High-sensitivity C-reactive protein predicts adverse outcomes after non-ST-segment elevation acute coronary syndrome regardless of GRACE risk score, but not after ST-segment elevation myocardial infarction. Rev Port Cardiol 2013;32:117-22. [PubMed]
- Deraz TE, Kamel TB, El-Kerdany TA, et al. High-sensitivity C reactive protein as a biomarker for grading of childhood asthma in relation to clinical classification, induced sputum cellularity, and spirometry. Pediatr Pulmonol 2012;47:220-5. [PubMed]
- Chiesa C, Signore F, Assumma M, et al. Serial measurements of C-reactive protein and interleukin-6 in the immediate postnatal period: reference intervals and analysis of maternal and perinatal confounders. Clin Chem 2001;47:1016-22. [PubMed]
- Ambalavanan N, Ross AC, Carlo WA. Retinol-binding protein, transthyretin, and C-reactive protein in extremely low birth weight (ELBW) infants. J Perinatol 2005;25:714-9. [PubMed]
- Kasper DC, Mechtler TP, Reischer GH, et al. The bacterial load of Ureaplasma parvum in amniotic fluid is correlated with an increased intrauterine inflammatory response. Diagn Microbiol Infect Dis 2010;67:117-21. [PubMed]


