Metachronous lung cancer that presented as bilateral synchronous lung cancer
Introduction
A primary lung cancer that develops following curative treatment of another primary lung cancer is referred to as metachronous lung cancer (MLC). Thus, every patient who undergoes curative treatment for primary lung cancer is a potential candidate for MLC. On the other hand, the presence of a second primary lung cancer at the time of diagnosis in a patient with primary lung cancer is referred to as synchronous lung cancer (SLC) (1).
Case presentation
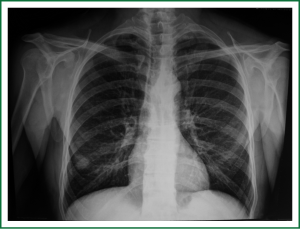
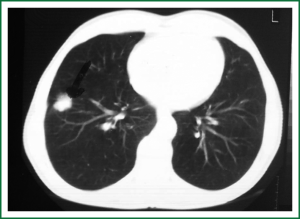
In 2005, a 2-cm non-homogeneous nodule in the right inferior lobe was observed on a chest radiogram (Figure 1) of a 62-year-old male patient who presented with right-sided pain and smoked 50 packs of cigarettes a year. Computed tomography (CT) revealed a nodule with spicular edges in the anterobasal segment of the inferior lobe (Figure 2). Trans-thoracic fine needle biopsy (TTFNB) was performed, and squamous cell cancer was diagnosed. Positron emission tomography (PET) revealed a standard uptake value (SUV) of 9.2 for the nodule, and pathological uptake was not observed in any other region or in the mediastinum. Distant metastasis was absent. The patient underwent a thoracotomy with right inferior lobectomy and mediastinal lymph node dissection (MLND). The pathology results confirmed T1N0M0R0 squamous cell cancer (T diameter: 2.5 cm).
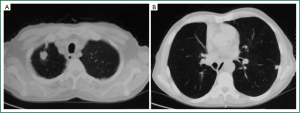
The patient was scheduled for a follow-up program and was monitored by physical examination, posteroanterior (PA) chest X-ray, and thoracic CT for signs and symptoms every 6 months during the first 5 years and annually thereafter. PA chest X-ray and thoracic CT obtained during the 72-month postoperative follow-up visit showed two nodules: one in the right apical region, measuring 2.9 cm × 1.8 cm, and one in the left inferior lobe, measuring 1.1 cm × 1 cm (Figure 3). PET-CT was performed. The SUV of the lesion in the right apical region was 8, and that of the left inferior lobe was 6. No endobronchial lesions were observed by fibreoptic bronchoscopy. TTFNB of the lesion in the right apical region revealed adenocarcinoma. Cranial magnetic resonance imaging (MRI) revealed no pathology. On pulmonary function tests, the FEV1 was 1.96 (73.6%), and the FEV1/FVC was 75.1.
During mediastinoscopy, punch biopsies were taken from lymph nodes 2R, 2L, 4R, 4L, and 7, but N2 disease was not noted. This case was considered to involve a bilateral synchronous tumour. Wedge resection via video-assisted thoracic surgery was performed on the undiagnosed nodule in the left inferior lobe, and the specimen was sent for frozen section examination. When the result came back as non-small cell lung cancer, the patient then underwent superior segmentectomy of the left inferior lobe + MLND in the same session. The pathology results confirmed T1aN0M0 adenocarcinoma (70% solid, 30% acinary; T diameter: 1.2 cm × 0.7 cm). Four weeks later, thoracotomy and apical segmentectomy + MLND were carried out for the nodule in the right superior lobe, which had been previously diagnosed. The pathology results confirmed T1bN0M0 adenocarcinoma (60% solid, 40% acinary; T diameter: 3 cm × 2.5 cm × 2 cm).
The patient received adjuvant chemotherapy in the postoperative period and is currently under an uneventful follow-up (87 months since the first operation and 15 months after the operations for the metachronous cancers).
Discussion
A newly developed lung cancer in a patient who has undergone curative treatment for primary lung cancer may represent either metastasis of the first cancer or MLC (1,2). In our case, MLC was diagnosed because the first cancer was squamous cell cancer, while the second was adenocarcinoma.
SLC is defined as the presence of a second primary lung cancer at the time of diagnosis in a patient with a primary lung cancer (1). When there are more than one primary cancer with the same histopathological type in a patient at the time of diagnosis, either the cancers are SLCs that developed independently or one of them is the primary tumour and the other is a metastasis of the primary tumour. The most reliable diagnostic method is comparison of the molecular and genetic features of the tumours. However, as this can be done in very few centres, patients can be assessed based on clinical criteria such those of Martini and Melamed (1,2). In the present case, bilaterally located tumours that developed during the follow-up period were considered to be SLCs because of the absence of mediastinal or systemic metastasis.
SLC is not considered to be a separate entity according to the 7th edition of the AJCC Lung Cancer Staging criteria. M1a disease is defined as separate nodules in a contralateral lobe. However, the assessment with regard to M1a was carried out in 369 cases of bilateral SLC, only seven of which underwent bilateral surgical treatment (3). Therefore, considering such cases as M1a disease is open for debate. In recent years, the number of publications on SLC has increased, and survival is satisfactory in the majority of these cases (4).
Curative surgical resection is recommended in patients with MLC. Surgical treatment of MLC depends on the side and size of the newly developed tumour, the surgical procedure performed on the first tumour, and the patient’s pulmonary function (1). The mean 5-year survival in patients with MLC is 20%, while that in patients who can undergo resection is 36% (20-50%) (1,2). Indications for adjuvant treatment after surgical resection are similar to those for other patients (5).
We concluded that the most appropriate mode of treatment for the MLC that developed in our patient was surgical resection. We performed bilateral sub-lobar resection because of the small sizes of the tumours, the need to perform bilateral resection, and the patient’s pulmonary function capacities. Based on the reports on the relationship between postoperative adjuvant treatment and better survival rates in patients with SLC, neoadjuvant treatment was given to our patient.
A case of MLC that developed as SLC has not been reported elsewhere in the English-language literature. We believe that this rare case and the assessment of our approach to the patient merit attention.
Acknowledgements
Disclosure: The authors declare no conflicts of interest and had no financial assistance in the writing of this manuscript.
References
- Shen KR, Meyers BF, Larner JM, et al. Special treatment issues in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:290S-305S.
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [PubMed]
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260-71. [PubMed]
- Rami-Porta R, Giroux DJ. Goldstraw P. The new TNM classification of lung cancer in practice. Available online: http://www.pneumonologia.gr/articlefiles/BREATHE%20TNM%20in%20clinical%20practise.pdf
- NCCN clinical practice guidelines in oncology (NCCN Guidelines), Version 2.2012. Available online: www.nccn.com