False positive hepatitis C antibody test results in left ventricular assist device recipients: increased risk with age and transfusions
Introduction
Left ventricular assist devices (LVADs) are increasingly accepted as destination therapy or as a bridge to cardiac transplantation (1-4). At the time of transplantation, 50–60% of patients have an LVAD and 3% have a right ventricular assist device (RVAD) (5). Allosensitization has become an increasing concern with the use of ventricular assist devices. Although there is limited literature to explain this phenomenon, development of anti-HLA antibodies has been well documented amongst the pediatric and adult cardiac transplant population (6,7). Other sensitizing events that may occur prior to transplantation include pregnancy, transfusion of blood products or prior organ transplantation.
Itescu et al. reported that the direct contact between the LVAD and blood cells, much like that seen in hemodialysis, results in significant changes in systemic immunologic and thrombostatic functions (8-10). Monocyte-T-cell-interactions occurring on the LVAD surface may result in aberrant T-cell activation and proliferation. Additionally, B-cell hyperreactivity may occur, resulting in significantly higher frequencies of autoantibodies, including circulating antiphospholipid and anti-HLA antibodies (8-10).
There have been recent reports of false positive hepatitis C (FPHC) serology after LVAD placement. Srivastava et al. (11), Sindermann et al. (12), Durand et al. (13), and most recently Heinrichs et al. (14) reported FPHC developing in 30%, 16%, 40%, and 59% of patients undergoing LVAD implantation, respectively. There is little published data explaining the pathophysiology of these false-positive results or describing the characteristics of the patient populations with FPHC serology results. This study aimed to determine the prevalence of FPHC results in patients who received LVAD at our institution, and describe the clinical and laboratory characteristics of these patients.
Methods
Study design
This is a retrospective study that was conducted at Montefiore Medical Center, a 1,490-bed tertiary care center that serves as the University Hospital of the Albert Einstein College of Medicine. Our study was approved by the Institutional Review Board (IRB) of Montefiore Medical Center (12-12-402). The objective was to describe clinical and laboratory characteristics of those LVAD recipients who developed FPHC antibody tests and those who did not.
Patients included in the study were adults 18 years of age or older who had consecutively undergone LVAD placement at our institution between January 2007 and January 2010, and were bridged to heart transplantation by January 2013. Patients who had received earlier generation LVAD devices (e.g., Heartmate XVE) and who did not undergo complete hepatitis C testing were excluded. Paper charts and electronic medical records of the patients were evaluated to obtain patient, LVAD, and transplantation characteristics. Characteristics included patient age, gender, type of LVAD, days from LVAD to heart transplantation, and blood product transfusions. Transfusion records were obtained using the SafeTrace Tx Transfusion Management Software System (Braintree, MA, USA) and electronic medical records.
Laboratory tests reviewed included albumin, globulin, serum protein electrophoresis, rheumatoid factor, antinuclear antibody, hepatitis C serology, and panel reactive anti-HLA antibody (PRA).
Testing and definitions
Hepatitis C virus (HCV) antibody testing by enzyme-linked immunosorbent assay (ELISA, using ADVIA Centaur XP, Siemens) was routinely performed before and after LVAD placement. Patients with positive serology for hepatitis C antibody underwent further confirmation with a Hepatitis C Virus RIBA test (Chiron RIBA HCV 3.0 Strip Immunoblot Assay, Novartis Vaccines and Diagnostics) and/or Hepatitis C Virus RNA PCR test (bDNA System 440 Siemens from 2007 to 3/2010, Cobas AmpliPrep/Cobas TaqMan HCV from 3/2010 to 3/2014)). Prior to April 4, 2011, a nationwide shortage of reagent limited our ability to perform RIBA testing, and PCR testing was the only confirmation available thereafter. Those patients with positive ELISA results who subsequently had negative HCV RNA results were considered to have FPHC.
PRAs were routinely performed prior to LVAD implantation and between the time of device implantation and heart transplantation. Prior to 2009, sensitization to HLA antigens was assessed using complement-dependent cytotoxicity (CDC). Starting in 2009, patients’ sera were tested using Single Antigen Beads (SAB; One Lambda, Canoga Park, CA). Using SAB, PRA was calculated (cPRA) based on the specificity of the observed anti-HLA antibodies and the frequency of the target HLA antigens in the general population (optn.transplant.hrsa.gov/resources/allocation-calculators). Sensitization to HLA antigens was defined as cPRA >10%. Since the sensitivity of the SAB and CDC assays differs significantly, cPRA values determined prior to and post-VAD implantation were compared only in patients who were tested using the SAB assay at all times (n=20).
Statistical analysis
Data were analyzed with SPSS software (SPSS, IBM version 21, Chicago, IL) for all univariate tests. Results of continuous variables were expressed as mean ± standard deviation. Comparisons of continuous variables were done using student’s t-test or Mann-Whitney test. Categoric variables were compared using Pearson chi-square test or Fisher’s exact test. All variables demonstrating a P<0.10 in their associations with FPHC were entered into a multivariate logistic regression model with FPHC as the dichotomous outcome variable. The regression analysis utilized the Firth method in consideration of the small sample size (R version 3.3.2 for Windows). Statistical significance was defined as rejection of the null hypothesis if P<0.05.
Results
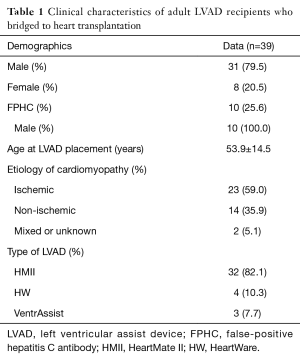
We identified 48 adult LVAD recipients who bridged to heart transplantation at our center from January 2007 to January 2013. We excluded five patients who did not have complete hepatitis C serologic testing and four patients who had received the earlier generation HeartMate XVE device. There were 39 remaining patients for study evaluation. The characteristics of these patients are listed in Table 1. Mean age at LVAD placement and heart transplantation was 55 and 56 years, respectively. Twenty-three (59%) had ischemic cardiomyopathy and 32 (82%) had HeartMate II LVAD implanted prior to transplantation. None of the patients had received more than one LVAD.
Full table
All 39 study patients had HCV antibody negative serologies prior to LVAD implantation. Ten (25.6%) of these patients were identified with positive hepatitis C antibody post-LVAD implantation. Of the 10 patients with FPHC, 8 had received Heartmate II LVADs and 2 had received VentrAssist devices. Among these 10 patients, the mean time to false positive serology was 163 days after LVAD implantation, with a range of 52 to 299 days. On further testing, seven of the ten patients with positive hepatitis C antibody had negative HCV PCR tests, two had negative HCV RIBA and negative HCV PCR tests, and one had negative HCV RIBA. RIBA patterns observed in three patients were compared and only one patient demonstrated antibodies to 5-1-1p/c100p. No antibodies to C33p, c22p, NS5, or hSOD were detected. After LVAD explantation at the time of heart transplantation, 9 of the 10 patients with FPHC were seronegative for hepatitis C and the tenth was negative for hepatitis C by PCR.
Clinical characteristics were compared between the 10 patients with FPHC and the 29 who did not develop hepatitis C antibody reactivity. Older age at LVAD implantation (P=0.002) was significantly associated with FPHC (Table 2). Although all of the PFHC events occurred in males, the association with male gender failed to achieve statistical significance (P=0.086). There was no correlation of gender, LVAD type, time between VAD placement and heart transplantation with FPHC.

Full table
We compared the number of blood transfusion units received by patients with and without FPHC. We found a significant univariate association between the development of FPHC and the number of units of red blood cells transfused between the time of LVAD implantation and either the FPHC result or the last negative hepatitis C test (P=0.0344). Fresh frozen plasma transfusion was not significantly associated (P≤0.01) with FPHC results.
The multivariate logistic regression model included age, blood transfusion units, and gender as covariates. The only variable found to have a significant association with FPHC in this analysis was age which demonstrated an adjusted odds ratio of 1.126 (95% CI: 1.013–1.302; P=0.022), equating to a 12.6% increase in odds of FPHC for each additional year of age.
As expected, LVAD implantation did result in a significant increase in the level of sensitization to HLA antigens. Anti-HLA antibody results obtained using the SAB assay pre- and post-LVAD (mean interval 11 months) were available in 20 patients. These results indicated that the number of sensitized patients increased from 1 (5%) pre-LVAD to 7 patients (35%) post-LVAD (P<0.0001). Out of 6 patients who developed anti-HLA antibodies post-LVAD, 3 patients displayed antibodies to both anti-HLA class I and class II, 2 patients developed only anti-HLA class I and one patient only class II antibodies. The reactivity pattern of these antibodies was consistent with that of “true” anti-HLA antibodies, showing specificity for discrete HLA antigens and/or cross-reactive HLA groups. Notably, none of the 7 patients with anti-HLA antibodies post-LVAD showed FPHC antibodies, suggesting no association between the development of anti-HLA antibodies and FPHC serology. We also compared the post-LVAD cPRA values in patients with and without FPHC. There was no significant difference between the cPRA values in the two groups (Table 2).
Mean measurements of rheumatoid factor (P=0.001) and globulin fraction (P<0.001) but not of antinuclear antibody or prealbumin, were significantly increased after LVAD implantation (data not shown). However, none of these significantly differed for those who developed FPHC and those who did not; additionally, anticardiolipin IgG or IgM, anti-neutrophil cytoplasmic antibody, and c-reactive protein did not differ (data not shown). Only one patient had an identified autoimmune disorder; this patient had systemic lupus erythematosus and did not develop FPHC.
Discussion
In this study, we found that 26% of LVAD recipients receiving the newer generation continuous flow devices (82% of which were HeartMate II LVADs) who were bridged to transplantation developed FPHC serology. These patients were older at the time of device placement (mean age 64 vs. 50 years among those with true negative hepatitis C testing). Additionally, a statistically significant association of red blood cell transfusion and FPHC was found on univariate analysis. However, a high transfusion rate may be a surrogate marker rather than a risk factor for FPHC. Although cPRA, globulin fraction, and rheumatoid factor were significantly increased post-LVAD, possibly reflecting a reaction to the LVAD, none of these correlated with the development of FPHC. Interestingly, no association was found between receipt of fresh frozen plasma and FPHC. Fresh frozen plasma contains coagulation factors, fibrinolytic and complement proteins; the passive transfer of antibodies or other plasma proteins in fresh frozen plasma could have been expected to interfere with hepatitis C antibody testing and cause FPHC results. The only radioimmunoblot band associated with FPHC in this and prior studies (11,13) was c5-1-1p/c100p.
Previously, Srivastava et al. reported on a cohort of 23 patients who underwent LVAD placement between 2006 and 2008, of whom 13 received the earlier generation HeartMate XVE and 11 received the HeartMate II device; 7 developed FPHC (11). Five patients with HeartMate II and 1 patient with HeartWare LVAD of a total of 37 patients had FPHC in the Sindemann report (12). Durand et al. reported on a cohort of 53 patients between 2005 and 2012, of whom 92% had received HeartMate II LVAD (13). In the Heinrichs review, 19 of 32 patients who received HeartWare devices between 2011 and 2015 developed FPHC (14). Unlike the Durand study, we did not observe the duration of LVAD to be predictive of FPHC; the lack of consistent periodic measurements between the time of LVAD implantation and explantation did not allow us to examine any possible association of duration with FPHC. Additionally, none of these studies reported on the effects of age and blood transfusion on FPHC.
The underlying pathophysiology for the development of hepatitis C antibody reactivity is unclear. Our study and the reports by Durand (13) and Heinrichs (14) note the resolution of FPHC with device explantation. Sensitization to HLA antigens caused by LVADs is well known (6). Inert materials can trigger inflammation, fibrosis, and coagulation; such responses likely contribute to the pathogenesis of thrombosis and systemic inflammation. Of interest is the recent report of increasing thrombosis associated with HeartMate II since 2011 (15). Possibly, changes in design, shear forces, and materials in LVADs may have some role in the development of FPHC serology. Yet the presence of the LVAD is not sufficient for the development of FPHC. Of interest is the finding by Srivastava (11), Sindemann (12), and Heinrichs (14) of the return to negative hepatitis C serologic status upon serial testing in several patients while the LVAD is in place. This suggests that the immunologic reaction to the LVAD may not be constant but may be intermittent and susceptible to other undefined factors. Possibly, the FPHC rates here and previously published are underestimates, as testing captures information at discrete time points only. It would be of interest to follow serial hepatitis C serologies after transplantation. Previous studies have not determined an association of FPHC serology with age, gender, or device type. While anti-HLA antibodies are elevated in recipients of LVADs, there is no association of these antibodies with FPHC serology. Our study is the first, however, to suggest an association of FPHC with age and increase red blood cell transfusions. These may reflect as yet undetermined host and inflammation factors.
There are a number of potential limitations to this study. The retrospective nature of the review may have allowed unrecognized bias. The study is based on data from a single large tertiary care teaching hospital; however, similar rates of FPHC reactivity have been reported elsewhere. The restriction of FPHC to males in our sample is interesting, but may require a larger sample in order to assess the importance, or lack thereof, of this observation. The physical properties of the devices evaluated, including materials and surfaces, differ from one another and may differ in their ability to cause allosensitization. Patient characteristics before and after LVAD implantation and after heart transplantation may have differed in unrecognized ways. Testing for hepatitis C was not performed at the same time points after LVAD implantation for all patients; possibly, more frequent testing would have uncovered more FPHC or better illustrated the intermittent nature of FPHC. Information regarding blood transfusions received prior to LVAD placement and outside of our institution were not available for inclusion in data collection and analysis. PRA testing used at our institution became more sensitive in 2009 and testing comparisons were made only for those patients who were tested prior to and post-LVAD using Luminex Single Antigen assay. Patients who had incomplete hepatitis C antibody testing were excluded from the analysis, causing a possible underestimation of the problem. RIBA testing was not universally available for our patients.
Conclusions
Patients who have undergone LVAD implantation may show evidence of the development of hepatitis C antibody reactivity which is not associated with detectable hepatitis C RNA or with radioimmunoblot reactivity and which apparently resolves with heart transplantation and LVAD explantation. Patients with FPHC tend to be older at the time of LVAD implantation and to have been more heavily transfused with red blood cells between the time of surgery and hepatitis C testing than were those with true negative hepatitis C testing. Clinicians should be aware of this increased risk of FPHC in older LVAD recipients and to promptly perform confirmatory testing in order to avoid unnecessary alarm, potential acceptance from high-risk donors, and possible delay in transplantation. Further studies should be done to better delineate the relationship between transfused blood components and immune activation, the effects of sensitization on antibody-mediated rejection and allograft vasculopathy, and to prospectively evaluate immunologic profiles of all patients undergoing LVAD implantation, especially with the later generation models.
Acknowledgements
The authors gratefully acknowledge Ryung S. Kim, PhD. for his assistance with the multivariate analysis.
Footnote
Conflicts of Interest: Presented in part at the 2013 American Transplant Congress Annual Meeting, Washington, May 18-22, 2013; Dr. Goldstein is a chair of the adverse event committee for a Terumo Inc. clinical trial, was on the board of Heartware Inc., and has received support for meeting travel from Thoractec Inc/St. Jude Inc. as a national clinical trial principal investigator.
References
- Park SJ, Milano CA, Tatooles AJ, et al. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Heart Fail 2012;5:241-8. [Crossref]
- Frazier OH, Rose EA, Macmanus Q, et al. Multicenter clinical evaluation of the HeartMate 1000 IP left ventricular assist device. Ann Thorac Surg 1992;53:1080-90. [Crossref]
- McCarthy PM, Portner PM, Tobler HG, et al. Clinical experience with the Novacor ventricular assist system. Bridge to transplantation and the transition to permanent application. J Thorac Cardiovasc Surg 1991;102:578-86; discussion 586-7.
- Oz MC, Argenziano M, Catanese KA, et al. Bridge experience with long-term implantable left ventricular assist devices. Are they an alternative to transplantation? Circulation 1997;95:1844-52. [Crossref]
- Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report--2010. J Heart Lung Transplant 2010;29:1089-103. [Crossref]
- Velez M, Johnson MR. Management of allosensitized cardiac transplant candidates. Transplant Rev (Orlando) 2009;23:235-47. [Crossref]
- O'Connor MJ, Harville TO, Rhodes-Clark B, et al. Quantification, identification, and relevance of anti-human leukocyte antigen antibodies formed in association with the berlin heart ventricular assist device in children. Transplantation 2013;95:1542-7. [Crossref]
- Itescu S, John R. Interactions between the recipient immune system and the left ventricular assist device surface: immunological and clinical implications. Ann Thorac Surg 2003;75:S58-65. [Crossref]
- Itescu S, Ankersmit JH, Kocher AA, et al. Immunobiology of left ventricular assist devices. Prog Cardiovasc Dis 2000;43:67-80. [Crossref]
- Itescu S, Schuster M, Burke E, et al. Immunobiologic consequences of assist devices. Cardiol Clin 2003;21:119-33. ix-x. [Crossref]
- Srivastava AV, Hrobowski T, Krese L, et al. High rates of false-positive hepatitis C antibody tests can occur after left ventricular assist device implantation. ASAIO J 2013;59:660-1. [Crossref]
- Sindermann JR, Holthaus AJ, Schepers M, et al. False-positive hepatitis C testing in long-term LVAD support. ASAIO J 2015;61:e19. [Crossref]
- Durand CM, Marr KA, Ostrander D, et al. False-positive hepatitis C virus serology after placement of a ventricular assistance device. Transpl Infect Dis 2016;18:146-9. [Crossref]
- Heinrichs A, Antoine M, Steensels D, et al. HCV false positive immunoassays in patients with LVAD: A potential trap! J Clin Virol 2016;78:44-6. [Crossref]
- Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33-40. [Crossref]


