Hormonal therapies in young breast cancer patients: when, what and for how long?
Introduction
Breast cancer (BC) in women <40 years is a rare condition (1) despite an increased incidence in premenopausal women has been recently reported in several countries. In the United States, 5.5% of BCs occur in women 2). While some preliminary data suggested a higher prevalence of triple negative and Human Epidermal Growth Factor Receptor 2 (HER-2)-positive disease and found an age-related expression of key BC-associated genes, when correcting for subtype and other significant clinico-pathologic features [estrogen receptor (ER) status and histologic grade] no gene differences were retained between age-defined groups (≤45 and ≥65 years) (3).
The choice of systemic treatment for invasive BC (both early and advanced disease) should not be age-specific but driven by the biological characteristics of the tumor (including hormone receptor status, HER-2 amplification, proliferation and grade), the tumor stage and patient’s comorbidities (4). Premenopausal women with invasive ER-positive (ER+) BC should be considered for adjuvant endocrine therapy (ET) regardless of age, lymph-node status or chemotherapy administration (5-7).
Several open questions of ET in premenopausal women with ER+ BC will be addressed:
- Is there a role for neoadjuvant endocrine therapy?
- Is there an optimal adjuvant endocrine therapy?
- Is there an optimal duration of endocrine therapy?
- What’s the role of ovarian function suppression/ablation?
- What’s the role of chemotherapy-induced-amenorrhea?
- What’s the role of aromatase inhibitors?
- What’s the impact of side effects?
- What’s the role of endocrine therapy in young women with metastatic breast cancer?
Five years of adjuvant tamoxifen, with or without a luteinizing hormone-releasing hormone (LH-RH) agonist, is considered standard ET for premenopausal women (5,7,8). Based on the efficacy shown in postmenopausal women (9), adjuvant aromatase inhibitors (AIs) in combination with ovarian function suppression (OFS) have been investigated in premenopausal patients with early BC. The Austrian Breast and Colorectal Cancer Study Group Trial 12 (ABCSG-12) has shown that 3-year adjuvant therapy with anastrozole plus goserelin provides a comparable disease-free survival (DFS) to that associated with tamoxifen plus goserelin (10). The upcoming results of the Suppression of Ovarian Function Trial (SOFT) (11) and Tamoxifen and EXemestane Trial (TEXT) (12) trials will provide additional evidence on the role of adjuvant AIs, if any, in premenopausal BC patients.
The optimal duration of adjuvant ET in young women remains uncertain. Recent data from the ATLAS and aTTom studies suggest that continuing tamoxifen to 10 years rather than stopping at 5 years gives a further reduction in recurrence and mortality, particularly after year 10 (13,14). Neo-adjuvant ET in premenopuasal patients has never been adequately studied.
As recommended for early BC, also in the metastatic setting age alone should not be a reason to prescribe more aggressive therapy: ET is the preferred option for ER+ disease, unless there is evidence of endocrine resistance or need for rapid disease and/or symptom control (15,16).
In young patients with ER+ metastatic breast cancer, tamoxifen in combination with OFS or ovarian ablation (OA) is the 1st-line ET of choice (17). AIs together with OFS/OA can be considered after progression on tamoxifen and OFS/OA (18,19). Fulvestrant has not yet been studied in pre-menopausal women (20,21).
In endocrine-responsive metastatic BC, most studies addressing the combination of ET and chemotherapy showed an increased overall response rate (ORR) or an increased time to progression (TTP) but no improvement in overall survival (OS) (22).
Neoadjuvant endocrine therapy
Neoadjuvant ET should not be proposed to young women outside clinical trials (4). Preliminary data suggested that neoadjuvant ET can be effective in premenopausal women (23). The use of letrozole and a LH-RH agonist as primary therapy was investigated in 32 premenopausal women with ER+ BC. The ORR was 50% (95% CI, 32-68%) and no patient progressed during treatment. Response was significantly associated with younger age (P24). In the STAGE study, in 204 patients treated with 24 months’ neoadjuvant therapy with goserelin plus anastrozole or tamoxifen, the combination with anastrozole achieved a significantly better ORR than goserelin plus tamoxifen (70.4% vs. 50.5%; 95% CI, 6.5-33.3; P=0.004) (25). The ORR achieved by the anastrozole group compares favorably to the ORR achieved with chemotherapy in luminal B patients (26) but a definitive randomized trial is warranted.
Tamoxifen
Tamoxifen, a selective ER modulator (SERM), is a prodrug metabolized by CYP3A4 and CYP2D6 into two active hydroxylated metabolites, 4-hydroxytamoxifen and 4-OH-N-des-methyltamoxifen (endoxifen). Endoxifen affinity for ER is about 100 times greater than tamoxifen. The effects on BC cells are produced by inhibition of both translocation and nuclear binding of the ER (27).
The benefits of adjuvant tamoxifen have been repeatedly demonstrated by the meta-analyses of the Early Breast Cancer Trialists Collaborative Group (EBCTCG). The latest overviews showed a substantial benefit both in premenopausal and postmenopausal women with ER+ BC regardless of age or the use of chemotherapy (28-30). In the 2011 overview, with a median follow-up of 13 years (30), 5 years of tamoxifen compared to no ET was associated with a reduction in BC recurrence by 39% [relative risk (RR) for recurrence 0.61, 95% CI, 0.57-0.65]. This translated into a 13% absolute reduction in the risk of recurrence at 15 years (33% versus 46%). The impact on disease recurrence was mainly seen in the first 5 years whereas the mortality reduction was significant throughout the first 15 years. A 9% absolute reduction in BC-related death was observed at 15 years (24% versus 33%), and the risk of BC mortality was reduced by 30% (RR for death 0.70, 95% CI, 0.64-0.75). No effect of tamoxifen was reported for ER-negative disease. The magnitude of benefit was greater for women with node-positive disease and risk reductions were similar for younger as compared to older women. Several cooperative groups also reported similar benefits of adjuvant ET in very young (<35 years) women as compared to older premenopausal women because of the lower rate of permanent amenorrhea following adjuvant chemotherapy in this population (31-34).
Overall, approximately 20% of ER+ BCs are progesterone receptor (PgR)-negative: these tumors are known to have a worse prognosis than the PgR-positive counterparts (35) but the proportional benefit with tamoxifen is the same as for PgR-positive cancers (30).
HER-2 overexpression is also associated with an adverse prognosis (36). Data on HER-2 influence on adjuvant ET in younger women are limited, but in the presence of oophorectomy, the impact of adjuvant tamoxifen on outcome is comparable in patients with HER2-positive and HER2-negative tumors (37).
An association between CYP2D6 genotype and tamoxifen metabolism influencing anti-tumour activity was investigated in >20 published studies with highly inconsistent results (38). At present, CYP2D6 pharmacogenetic driven treatment decisions cannot be recommended outside clinical studies.
Is there an optimal duration of endocrine therapy?
The duration of ET has not been adequately studied in young women and is still a matter of debate. The recently published ATLAS trial included 15.244 pre- and postmenopausal women (13). Six-thousand-eight-hundred-forty-six women with ER+ disease who received tamoxifen for 5 years were randomized to continue for another 5 years (continuers group) or to stop (control group). With a median follow-up of 7.6 years, continuing tamoxifen reduced the risk for BC recurrence, compared to a 5-year treatment course (18% versus 21%, RR 0.84, 95% CI, 0.76-0.94). A persistent and more significant effect was found after year 10 (RR 0.90, 95% CI, 0.79-1.02 during years 5-9 and 0.70, 95% CI, 0.62-0.90 during subsequent years). The effect was independent of age (10% of patients were premenopausal at study entry) and nodal status (41% of patients were node-positive at diagnosis). A significant reduction in BC mortality (331 vs. 397 deaths; P=0.01), and overall mortality (639 vs. 722 deaths; P=0.01) were reported. There was a 29% reduction in the risk of BC mortality after year 10 (RR 0.71, 95% CI, 0.58-0.88). Longer therapy was associated with an increased risk for pulmonary embolism (RR 1.87, 95% CI, 1.13-3.07, P=0.01), and endometrial cancer (RR 1.74, 95% CI, 1.30-2.34, P=0.0002) with lower risk in premenopausal women, but no increase in the incidence of stroke (RR 1.06, 95% CI, 0.83-1.36), and a decrease in the incidence of ischemic heart disease (RR 0.76, 95% CI, 0.60-0.95, P=0.02).
Smaller trials in the past didn’t suggest any benefit to extend tamoxifen treatment (39,40) but these negative results could have simply been due to the play of chance because of the small numbers of patients recruited.
The UK adjuvant aTTom trial randomly allocated 7,000 women, most with unknown ER status, to continue tamoxifen to 10 years or stop at 5 years: the recently reported findings confirm the ATLAS reduction in recurrence and death from breast cancer (14).
Extending tamoxifen therapy beyond 5 years should be therefore considered in premenopausal women at high risk for late relapse (i.e., pathologically involved nodes, bigger tumors size, higher tumor grade) taking into account quality-of-life issues. In the ATLAS trial 84% of women allocated to continue were still on tamoxifen 2 years after entry (i.e., at year 7 after diagnosis). On the other hand, definite long-term side effects of tamoxifen do exist, which require longer follow-up and meta-analyses of all relevant trials for balanced risk/benefit evaluation.
The NCIC-CTG MA.17/BIG 1-97 trial reported a significant advantage to extended adjuvant ET with 5 years of letrozole in postmenopausal women with ER+ tumors, who had received 5 years of tamoxifen (41). A further analysis reported that premenopausal women at initial BC diagnosis, who became definitively postmenopausal at the time of randomization after 5 years of adjuvant tamoxifen, derived significantly more benefit in terms of DFS, from the extended therapy [hazard ratio (HR) =0.25, 95% CI, 0.12-0.51] than women who were postmenopausal at initial diagnosis, independent of nodal status (42).
Ovarian function suppression/ablation
OFS/OA can be achieved by surgery, radiation, chemotherapy, or LH-RH agonists. If ovarian targeted therapy is given, there is no available evidence favoring a specific form of ovarian function manipulation.
The EBCTG overview demonstrated that OA (by surgery or irradiation) or OFS with a LH-RH agonist significantly reduce the risk of recurrence and BC mortality in women <50 years with ER+ or ER-unknown early BC (29). However, the effects appear smaller in women who also received chemotherapy, probably because chemotherapy-induced amenorrhea (CIA) attenuated any additional effect of OFS/OA. In addition, the analysis may slightly underestimate the effects of ovarian treatment since 26% of women had ER-unknown disease, a proportion of whom had reasonably ER- disease.
The impact of adding OFS to adjuvant chemotherapy was studied in the ECOG-led Intergroup 0101 trial, where in premenopausal women with ER+ node-positive early BC the addition of both tamoxifen and goserelin improved DFS as compared to chemotherapy alone but no significant effect on DFS was shown with the addition of goserelin alone. A trend to DFS benefit from addition of goserelin to chemotherapy was demonstrated in an unplanned retrospective analysis of women 43).
Likewise, the International Breast Cancer Study Group (IBCSG) trial VIII randomized premenopausal women with node-negative ER+ early BC to either adjuvant CMF, goserelin for 2 years, or CMF followed by goserelin for 18 months. The addition of goserelin resulted in a small improvement in 5-year DFS that did not reach statistical significance (HR 0.80; 95% CI, 0.57-1.11). In an unplanned analysis according to age the subgroup of women <40 years derived a significant benefit (HR 0.34; 95% CI, 0.14-0.87) (44).
A subsequent larger EBCTG meta-analysis looked only at trials with known ER status and LH-RH agonists as method of OFS (45). OFS proved to be beneficial whether used alone (recurrence risk reduction of 28%, P=0.08), in addition to tamoxifen or chemotherapy (recurrence risk reduction of 13%, P=0.02), and as an alternative to chemotherapy. The effects of LH-RH agonists were greater in women <40 years in whom chemotherapy is less likely to induce permanent amenorrhea. However, there were few trials testing the addition of LH-RH agonists to tamoxifen (with or without chemotherapy) and no trials had compared a LH-RH agonist against chemotherapy with tamoxifen in both arms. Additionally, modern standard chemotherapies are generally less associated with premature menopause than those included in the overview and the question of whether adding a LH-RH agonist is only useful when amenorrhea is not achieved with chemotherapy is still unanswered.
Optimal duration of LH-RH agonists is also unknown, although most studies have utilized 2-3 years of LH-RH agonists with 5 years of tamoxifen.
In patients who do embark on OFS using LH-RH agonists, OFS is not always successfully achieved: cessation of menses alone is insufficient to confirm suppression, estradiol assays are often not standardized and problematic in terms of accuracy and interpretation in presence of very low levels of estradiol (46). Overall, the data available show biochemical suppression for the majority of patients but samples sizes are small and there is no data on the long-term maintenance of estradiol suppression. A pooled analysis of 193 premenopausal women with advanced BC treated with goserelin from 29 European trials showed incomplete menstrual suppression in 5% of patients (47). Anastrozole in combination with goserelin was not able to steadily suppress estradiol serum levels to the postmenopausal range in one third of 32 premenopausal patients with metastatic BC. Estradiol levels were not available in the remaining patients beyond 6 months to assure long-term suppression (18). Despite the reported limitations, estradiol levels should be checked on a regular basis (at least every 6 months), always in the same laboratory and preferably in a central reference laboratory.
When pharmacological suppression is chosen, monthly injection is the recommended way of administration, as tested in nearly all of the available trials.
The reversible OFS with LH-RH agonists can be particularly attractive to younger women, especially to those who did not complete childbearing before diagnosis, but the long-term risk of recurrence in ER+ BC should be taken into account when planning adjuvant ET in young patients (48).
Although the standard of care for ER+ premenopausal BC remains tamoxifen alone for 5 years, prospective trials to address the added role (if any) of OFS compared with tamoxifen alone have been undertaken (49). The SOFT trial will assess the role of OFS/OA in combination with the AI exemestane, compared with either OFS plus tamoxifen ortamoxifen alone. Three-thousand-sixty-six women were randomized into this study, which completed accrual in January 2011 (11). The TEXT trial assesses a LH-RH agonist with the addition of either tamoxifen or exemestane for 5 years (chemotherapy is optional): accrual of 2,672 women was completed in March 2011 (12). In the initial 890 patients entered in the TEXT trial, chemotherapy was chosen for 64% of patients, lymph node status being the predominant determinant of chemotherapy use (88% of node-positive versus 46% of node-negative) (50). The results of both these IBCSG-led trials, awaited in the course of 2014, will help the selection of the optimal ET for premenopausal women with ER+ early BC.
The role of surgical OA has been reconsidered with the advent of BRCA1/2 mutation testing in very young women or in patients belonging to hereditary BC families. Prophylactic oophorectomy is known to significantly reduce the risk of developing both breast and ovarian cancer in mutation carriers (51,52), who are often identified at the time of BC diagnosis. Prophylactic oophorectomy may therefore be considered when discussing adjuvant ET in this subgroup of young women.
The role of chemotherapy-induced-amenorrhea
In addition to direct cytotoxicity, adjuvant chemotherapy has an indirect endocrine effect in ER+ BC through the induction of OFS. Amenorrhea, even if transient (53), has been associated with improved treatment outcome in several trials (54).
In the IBCSG trial 13-93 of adjuvant chemotherapy ± tamoxifen in premenopausal node–positive women, patients with ER+ disease who experienced CIA had a significantly improved outcome (HR for amenorrhea vs. no amenorrhea =0.61; 95% CI, 0.44 to 0.86; P=0.004), whether or not they received tamoxifen (34).
In the NSABP trial B-30 in node-positive patients treated with both adjuvant anthracycline- and taxane-containing regimens, premenopausal women with ER+ tumors who had amenorrhea for ≥6 months after completion of chemotherapy had a significantly better survival (HR for death 0.52, P=0.002) and lower disease recurrence and second malignant incidence (HR 0.51, P<0.001) than those with no amenorrhea. By contrast, women with ER- tumors had a similar outcome regardless of whether they had or not amenorrhea (55,56).
The risk of CIA depends on the given regimen, total dose, dose-intensity, treatment duration, patient’s age, and patient’s ovarian reserve at the time of treatment initiation (54,57,58). The greatest risk is in women >40 years treated with alkylating agents (i.e., cyclophosphamide) but patient age also predicts amenorrhea in women treated with anthracycline-containing regimens (Table 1). The individual impact of taxanes on permanent amenorrhea is difficult to evaluate since they are usually administered sequentially or concurrently with anthracyclines and cyclophosphamide.
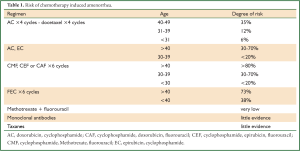
Full Table
In the NSABP B-30 trial, four cycles of AT (doxorubicin plus docetaxel), a regimen not containing cyclophosphamide, resulted in the lowest rate of amenorrhea (59).
The limited evidence available on the addition of trastuzumab to anthracyclines and/or taxanes shows no apparent increase in the rate of permanent amenorrhea.
Aromatase inhibitors
AIs are contraindicated in premenopausal patients because the suppression of peripheral aromatase results in reduced feedback to the hypothalamus and consequent ovarian stimulation (60). As a consequence, AIs must be used with great caution also in premenopausal women who have had CIA (61), because they can be associated with return of ovarian function and pregnancy, even in the absence of menses (62).
The ABCSG-12 trial randomized premenopausal women with ER+ early BC to receive 3 years of OFS with goserelin combined with either tamoxifen or anastrozole. Eligible patients had favorable prognosis (75% had T1, G1-2 tumors, only 30% had node-positive disease) and none received adjuvant chemotherapy (5% did receive neoadjuvant chemotherapy). At a median follow-up of 62 months, >2 years after treatment completion, there was no difference in DFS between patients on tamoxifen versus anastrozole (HR 1.08, 95% CI, 0.81-1.44; P=0.591), but OS was worse with anastrozole than with tamoxifen (HR 1.75, 95% CI, 1.08-2.83; P=0.02) (10). This latter observation could be partially related to the fact that, after disease recurrence, women receiving tamoxifen were more likely to be switched to an AI than those in the anastrozole group (61% versus 41%, respectively) who were switched to second-line, non-aromatase inhibitors, ET. In addition, overweight patients (BMI ≥25 kg/m2) treated with anastrozole had a nearly 50% increase in the risk of disease recurrence (HR 1.49; 95% CI, 0.93-2.38; P=0.08) and a three-fold increase in the risk of death (HR 3.03; 95% CI, 1.35-6.82; P=0.004) compared with patients treated with tamoxifen (63). Anastrozole efficacy might in fact be affected by an increased total-body aromatization in the fat tissue, in which precursors are metabolized to estrogens by the enzyme aromatase and subsequent incomplete suppression of estrogen production in peripheral body fat. Of note, >90% of patients remained disease-free, suggesting that combined adjuvant ET without chemotherapy, in appropriately selected premenopausal women with endocrine-responsive tumors, can be effective. A word of caution should be raised in very young women, as only 18% of women were ≤40 years of age when randomized. The upcoming results of the SOFT and TEXT trials will provide further insight into this clinically important question.
Side effects of endocrine therapy
Side effects of tamoxifen and OFS/OA mimic menopausal symptoms, including hot flashes, sweats, weight gain and sexual dysfunction, which may negatively impact quality of life. In addition, tamoxifen has both estrogen agonist and antagonist properties with different side effect profile depending on the target organ. Hot flashes are the most common side effect of tamoxifen, reported in up to 80% of women (64). Non-hormonal and non-pharmacological therapies such as phytotherapy or acupuncture can be effective to reduce the intensity of symptoms as well as low-dose antidepressants, pregabalin and gabapentin (65-67).
The estrogen-like effect of tamoxifen on the uterus may induce endometrial hyperplasia and endometrial tumors (68,69). In the most recent EBCTCG overview, 5 years of tamoxifen were associated with a low overall incidence of uterine cancer (3.8% percent versus 1.1% in the control group) only in women aged 55 to 69 years, with no impact on mortality from uterine cancer (30). To date, no evidence-based recommendations for routine screening (i.e., transvaginal ultrasound or endometrial biopsy) in women assuming tamoxifen were published. However, abnormal bleeding should be promptly investigated and expert opinion recommendations suggest annual gynecologic examinations (7).
As previously mentioned, in the 2011 EBCTCG meta-analysis women who received tamoxifen had an increased thrombo-embolic risk (30). Similar findings were reported in BC prevention trials (70,71). Tamoxifen is therefore contraindicated in women with prior history of deep-vein thrombosis and pulmonary embolism.
Patients should be informed of the possibility of getting pregnant while on tamoxifen, despite developing amenorrhea: the relatively high frequency of severe congenital abnormalities mandates a reliable non-hormonal contraception (72).
Tamoxifen and LH-RH agonists produce hypoestrogenism with associated hyperandrogenism, which could lead to specific side effects like hair loss (73,74).
On the other hand, tamoxifen may also increase plasma estradiol concentrations by interfering with the normal negative pituitary feedback mechanisms: the resulting FSH rise drives ovarian steroidogenesis and increased incidence of ovarian cysts (75). Endogenous sex hormone levels were not correlated with outcome in a high-risk postmenopausal population prevention trial (76) but further studies may be required to explore the potential impact on the breast of increased serum estrogen levels in premenopausal BC patients treated with tamoxifen.
While in postmenopausal women tamoxifen has a well-established agonistic estrogenic effect in bone, there is some evidence that tamoxifen may decrease bone mineral density (BMD) in premenopausal women, although the exact mechanism remains unclear. In the ZIPP (Zoladex in Premenopausal Patients) trial comparing different adjuvant ETs in early BC, a significant decline in BMD was seen after 2 years of treatment in patients receiving tamoxifen alone (77). In a Finnish survey in 111 premenopausal women with early BC treated with adjuvant chemotherapy, tamoxifen was associated with bone loss in patients who continued to menstruate after adjuvant chemotherapy whereas prevented bone loss in women who developed CIA (78). BMD has therefore to be regularly checked in premenopausal women receiving ET for BC. Bisphosphonates, although not yet approved for this indication, can prevent cancer therapy-induced bone loss and improve BMD in premenopausal women receiving therapy for BC (79-81) and should be promptly introduced at first signs of significant bone loss. Regular exercise has also a positive impact on bone mineralization and stimulation of osteogenesis (82).
Tamoxifen may adversely affect cognition (83), although few specific investigations on this side effect have been conducted and none in young women. In the ZIPP trial (6 cycles of CMF ± 2 years of goserelin, goserelin plus tamoxifen, or tamoxifen), no effect of treatment on the patients’ self-evaluation of memory and concentration was shown (84). Cognitive function is being prospectively investigated in patients participating in the SOFT trial.
Treatment with LH-RH agonists is associated with menopausal side effects such as hot flushes and vaginal dryness (85) and with more severe sexual dysfunction than tamoxifen alone (86). In 293 patients enrolled in the ZIPP trial differential side effects of ET were evident only in patients who did not receive chemotherapy. Goserelin resulted in similar symptoms as CMF, whereas the side effects of tamoxifen alone were milder with the exception of vaginal discharge. Vaginal dryness from goserelin was mitigated by the addition of tamoxifen. After cessation of ET, side effects decreased in patients who had not received CMF, whereas patients treated with CMF reported ongoing problems at the 3-year follow-up (84).
Fertility issues, feasibility and safety of pregnancy should be addressed in every young patient with BC. Reproductive issues are of great importance to young women, in particular for those who did not complete their families before BC diagnosis (87). The risk of ovarian failure is associated with the chemotherapeutic agents used and patient age. In women <35 years, adjuvant chemotherapy is less likely to induce permanent amenorrhea (88,89). Fertility preservation has to be discussed early after diagnosis and patients should ideally be referred to a fertility specialist before starting therapy (88). Pregnancy following BC does not seem to negatively influence DFS or OS in ER+ premenopausal patients (90,91). The impact of a temporary interruption of adjuvant ET to allow pregnancy will be addressed in a phase II trial within the Breast International Group (BIG) and North American Breast Cancer Group (NABCG) collaboration.
Side effects of AIs when combined with LH-RH agonists are consistent with the known safety profiles for each of the agents administered (10,19).
Weight gain is often reported by women treated with tamoxifen and OFS. Randomized trials have not reported an excess in weight gain in patients treated with tamoxifen as compared to those who received placebo (92,93). In prevention trials, weight gain did not differ between anastrozole, tamoxifen and placebo (94,95).
Weight gain ≥10% after BC diagnosis was associated with a non-significant increased risk of death (HR, 1.15; 95% CI, 0.98-1.35) but not of BC-specific mortality (HR, 1.03; 95% CI, 0.84-1.26) in 12,915 patients with BC (diagnosed between 1990 and 2006) from 4 population-based prospective cohort studies examining the role of physical activity, BMI, dietary factors and interventions and quality of life in BC prognosis (96).
Overall, a woman must experience substantial weight gain before an increased risk of death is observed but normal weight women at BC diagnosis are at the highest risk of experiencing the negative effects of weight gain on overall mortality outcomes (HR, 1.24; 95% CI, 0.98-1.56). Several mechanisms have been postulated through which weight gain may influence survival, including enhanced conversion in fat tissue of androgens to estrogens (97). As a consequence, prevention of weight gain appears to be a sound public health goal for BC survivors (98).
Younger age was found in several observational studies to be a factor associated with lower rates of treatment compliance (99). In a cohort of 288 French women diagnosed with BC <40 years, 29.7% (95% CI, 24.1-36.4%) had discontinued tamoxifen after 2 years; after 3 years the proportion increased to 39.5% (95% CI, 32.9-47.0%) (100).
Endocrine therapy in metastatic breast cancer
In premenopausal women with ER+ metastatic BC, a variety of ET have proven to be effective (SERMs, OFS/OA ± tamoxifen or AIs, progestational agents (megestrol acetate) (101). A meta-analysis comparing LH-RH agonist ± tamoxifen showed that the outcomes were significantly improved in patients who received the combination (17). On the basis of these results, a LH-RH agonist plus tamoxifen is currently recommended as the standard therapy in advanced BC.
Based on the available evidence in postmenopausal women with recurrent BC (15), the role of AIs has also been studied in premenopausal women. In a prospective, single-arm, multicenter phase II trial, 32 premenopausal patients were treated with goserelin and anastrozole achieving a clinic benefit rate of 71.9%, similar to that observed with AIs in postmenopausal women (18). Other small Phase II studies confirm the efficacy of AIs as 1st- and 2nd-line treatment in combination with a LH-RH agonist (24,102-104). In a phase II parallel group study, at median follow-up of 27.4 months, there was no statistical difference in the median TTP between premenopausal patients receiving letrozole plus goserelin and postmenopausal patients treated with letrozole alone [9.5 months (95% CI, 6.4 to 12.1 months) vs. 8.9 months (95% CI, 6.4 to 13.3 months)] (19).
The combination of LH-RH agonists and AIs can therefore be considered in premenopausal women with ER+ metastatic BC after progression on tamoxifen plus OFS but the exact role of this therapeutic option requires further investigation in randomized trials.
Considering the documented efficacy of fulvestrant in postmenopausal patients (105), some studies have been conducted in premenopausal patients as well (20,21). Bartsch et al. demonstrated a clinical benefit rate of 58% with fulvestrant plus goserelin in 26 patients pretreated with tamoxifen and aromatase inhibitors in combination with goserelin: median TTP was 6 months (95% CI, 2.4-9.6 months) and OS 32 months (95% CI, 14.28-49.72 months), respectively (106).
In endocrine-responsive metastatic BC, most studies addressing the combination of ET and chemotherapy showed an increased ORR or an increased TTP but no improvement in OS with no age-related differences (22). Trials examining concurrent versus sequential treatment with ET and chemotherapy need therefore to be conducted (107).
No specific endocrine resistance mechanisms have been identified in premenopausal patients and all the compounds developed to revert endocrine resistance have been tested only in postmenopausal patients so far.
Conclusions
Current available therapies in ER+ early and advanced BC in young women either modulate estrogen receptor activity (tamoxifen) or decrease estrogen production (OFS/OA). In early BC, several questions still need to be answered: (I) the optimal duration of pharmacological OFS; (II) the value of sequential chemotherapy and OFS, particularly in those women who do not develop CIA; (III) the utility of combined ETs (e.g., OFS + tamoxifen or AIs) and (IV) the optimal ET and treatment duration in high-risk ER+ patients
In advanced disease, ET has a major role in the long-term control of indolent disease and most of the time is associated with an acceptable toxicity profile.
Better tools to manage early menopause signs/symptoms (e.g., BMD, cognitive problems, fertility impairment and sexual disturbances) and careful monitoring of late toxicities (e.g., second cancers) need to be routinely implemented and specifically investigated.
Individualized, multidisciplinary approaches are needed to best address the complex physical and psycho-social scenario of BC at young age in order to maximize BC cure while minimizing the impact of diagnosis and treatment in women with demanding social and family commitments.
Acknowledgements
Disclosure: The authors indicated no potential conflicts of interest.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Samphao S, Wheeler AJ, Rafferty E, et al. Diagnosis of breast cancer in women age 40 and younger: delays in diagnosis result from underuse of genetic testing and breast imaging. Am J Surg 2009;198:538-43. [PubMed]
- Anders CK, Fan C, Parker JS, et al. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol 2011;29:e18-20. [PubMed]
- Cardoso F, Loibl S, Pagani O, et al. The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer. Eur J Cancer 2012;48:3355-77. [PubMed]
- Aebi S, Davidson T, Gruber G, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2011;22 Suppl 6:vi12-24. [PubMed]
- Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 2003;21:976-83. [PubMed]
- NCCN. Breast Cancer. Version 2.2013. In National Comprehensive Cancer Network Guidelines, Edition 2013.
- Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736-47. [PubMed]
- Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 2010;28:509-18. [PubMed]
- Gnant M, Mlineritsch B, Stoeger H, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol 2011;12:631-41. [PubMed]
- Suppression of Ovarian Function Plus Either Tamoxifen or Exemestane Compared With Tamoxifen Alone in Treating Premenopausal Women With Hormone-Responsive Breast Cancer (SOFT). In ClinicalTrials.gov Identifier: NCT00066690.
- Triptorelin With Either Exemestane or Tamoxifen in Treating Premenopausal Women With Hormone-Responsive Breast Cancer (TEXT). In ClinicalTrials.gov Identifier: NCT00066703.
- Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013;381:805-16. [PubMed]
- Gray RG, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013; 31(suppl): abstr 5.
- Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast 2012;21:242-52. [PubMed]
- Cardoso F, Fallowfield L, Costa A, et al. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2011;22 Suppl 6:vi25-30. [PubMed]
- Michaud LB, Jones KL, Buzdar AU. Combination endocrine therapy in the management of breast cancer. Oncologist 2001;6:538-46. [PubMed]
- Carlson RW, Theriault R, Schurman CM, et al. Phase II trial of anastrozole plus goserelin in the treatment of hormone receptor-positive, metastatic carcinoma of the breast in premenopausal women. J Clin Oncol 2010;28:3917-21. [PubMed]
- Park IH, Ro J, Lee KS, et al. Phase II parallel group study showing comparable efficacy between premenopausal metastatic breast cancer patients treated with letrozole plus goserelin and postmenopausal patients treated with letrozole alone as first-line hormone therapy. J Clin Oncol 2010;28:2705-11. [PubMed]
- Robertson JF, Semiglazov V, Nemsadze G, et al. Effects of fulvestrant 250mg in premenopausal women with oestrogen receptor-positive primary breast cancer. Eur J Cancer 2007;43:64-70. [PubMed]
- Young OE, Renshaw L, Macaskill EJ, et al. Effects of fulvestrant 750mg in premenopausal women with oestrogen-receptor-positive primary breast cancer. Eur J Cancer 2008;44:391-9. [PubMed]
- Pritchard KI. Combining endocrine agents with chemotherapy: which patients and what sequence? Cancer 2008;112:718-22. [PubMed]
- Gazet JC, Ford HT, Gray R, et al. Estrogen-receptor-directed neoadjuvant therapy for breast cancer: results of a randomised trial using formestane and methotrexate, mitozantrone and mitomycin C (MMM) chemotherapy. Ann Oncol 2001;12:685-91. [PubMed]
- Torrisi R, Bagnardi V, Pruneri G, et al. Antitumour and biological effects of letrozole and GnRH analogue as primary therapy in premenopausal women with ER and PgR positive locally advanced operable breast cancer. Br J Cancer 2007;97:802-8. [PubMed]
- Masuda N, Sagara Y, Kinoshita T, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol 2012;13:345-52. [PubMed]
- Alba E, Calvo L, Albanell J, et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol 2012;23:3069-74. [PubMed]
- Osborne CK. Steroid hormone receptors in breast cancer management. Breast Cancer Res Treat 1998;51:227-38. [PubMed]
- Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 1998;351:1451-67. [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771-84. [PubMed]
- Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet 2000;355:1869-74. [PubMed]
- Colleoni M, Rotmensz N, Peruzzotti G, et al. Role of endocrine responsiveness and adjuvant therapy in very young women (below 35 years) with operable breast cancer and node negative disease. Ann Oncol 2006;17:1497-503. [PubMed]
- Goldhirsch A, Gelber RD, Yothers G, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr 2001;(30):44-51. [PubMed]
- International Breast Cancer Study Group, Colleoni M, Gelber S, et al. Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: International Breast Cancer Study Group Trial 13-93. J Clin Oncol 2006;24:1332-41. [PubMed]
- Arpino G, Weiss H, Lee AV, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst 2005;97:1254-61. [PubMed]
- Burstein HJ. The distinctive nature of HER2-positive breast cancers. N Engl J Med 2005;353:1652-4. [PubMed]
- Love RR, Duc NB, Havighurst TC, et al. Her-2/neu overexpression and response to oophorectomy plus tamoxifen adjuvant therapy in estrogen receptor-positive premenopausal women with operable breast cancer. J Clin Oncol 2003;21:453-7. [PubMed]
- Fleeman N, Martin Saborido C, Payne K, et al. The clinical effectiveness and cost-effectiveness of genotyping for CYP2D6 for the management of women with breast cancer treated with tamoxifen: a systematic review. Health Technol Assess 2011;15:1-102. [PubMed]
- Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst 2001;93:684-90. [PubMed]
- Stewart HJ, Forrest AP, Everington D, et al. Randomised comparison of 5 years of adjuvant tamoxifen with continuous therapy for operable breast cancer. The Scottish Cancer Trials Breast Group. Br J Cancer 1996;74:297-9. [PubMed]
- Jin H, Tu D, Zhao N, et al. Longer-term outcomes of letrozole versus placebo after 5 years of tamoxifen in the NCIC CTG MA.17 trial: analyses adjusting for treatment crossover. J Clin Oncol 2012;30:718-21. [PubMed]
- Higgins MJ, Liedke PE, Goss PE. Extended adjuvant endocrine therapy in hormone dependent breast cancer: the paradigm of the NCIC-CTG MA.17/BIG 1-97 trial. Crit Rev Oncol Hematol 2013;86:23-32. [PubMed]
- Davidson NE, O’Neill AM, Vukov AM, et al. Chemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: results from INT 0101 (E5188). J Clin Oncol 2005;23:5973-82. [PubMed]
- International Breast Cancer Study Group (IBCSG), Castiglione-Gertsch M, O’Neill A, et al. Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast cancer: a randomized trial. J Natl Cancer Inst 2003;95:1833-46. [PubMed]
- LHRH-agonists in Early Breast Cancer Overview group, Cuzick J, Ambroisine L, et al. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet 2007;369:1711-23. [PubMed]
- Dowsett M, Folkerd E. Deficits in plasma oestradiol measurement in studies and management of breast cancer. Breast Cancer Res 2005;7:1-4. [PubMed]
- Blamey RW, Jonat W, Kaufmann M, et al. Goserelin depot in the treatment of premenopausal advanced breast cancer. Eur J Cancer 1992;28A:810-4. [PubMed]
- Jatoi I, Anderson WF, Jeong JH, et al. Breast cancer adjuvant therapy: time to consider its time-dependent effects. J Clin Oncol 2011;29:2301-4. [PubMed]
- Griggs JJ, Somerfield MR, Anderson H, et al. American Society of Clinical Oncology endorsement of the cancer care Ontario practice guideline on adjuvant ovarian ablation in the treatment of premenopausal women with early-stage invasive breast cancer. J Clin Oncol 2011;29:3939-42. [PubMed]
- Regan MM, Pagani O, Walley B, et al. Premenopausal endocrine-responsive early breast cancer: who receives chemotherapy? Ann Oncol 2008;19:1231-41. [PubMed]
- Eisen A, Lubinski J, Klijn J, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case-control study. J Clin Oncol 2005;23:7491-6. [PubMed]
- Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol 2008;26:1331-7. [PubMed]
- Pagani O, O’Neill A, Castiglione M, et al. Prognostic impact of amenorrhoea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: results of the International Breast Cancer Study Group (IBCSG) Trial VI. Eur J Cancer 1998;34:632-40. [PubMed]
- Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol 2006;24:5769-79. [PubMed]
- Swain SM, Jeong JH, Geyer CE Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med 2010;362:2053-65. [PubMed]
- Swain SM, Jeong JH, Wolmark N. Amenorrhea from breast cancer therapy--not a matter of dose. N Engl J Med 2010;363:2268-70. [PubMed]
- Meirow D, Biederman H, Anderson RA, et al. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol 2010;53:727-39. [PubMed]
- Torino F, Barnabei A, De Vecchis L, et al. Recognizing menopause in women with amenorrhea induced by cytotoxic chemotherapy for endocrine-responsive early breast cancer. Endocr Relat Cancer 2012;19:R21-33. [PubMed]
- Ganz PA, Land SR, Geyer CE Jr, et al. Menstrual history and quality-of-life outcomes in women with node-positive breast cancer treated with adjuvant therapy on the NSABP B-30 trial. J Clin Oncol 2011;29:1110-6. [PubMed]
- Dowsett M, Folkerd E, Doody D, et al. The biology of steroid hormones and endocrine treatment of breast cancer. Breast 2005;14:452-7. [PubMed]
- Ortmann O, Pagani O, Jones A, et al. Which factors should be taken into account in perimenopausal women with early breast cancer who may become eligible for an aromatase inhibitor? Recommendations of an expert panel. Cancer Treat Rev 2011;37:97-104. [PubMed]
- Smith IE, Dowsett M, Yap YS, et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: caution and suggested guidelines. J Clin Oncol 2006;24:2444-7. [PubMed]
- Pfeiler G, Königsberg R, Fesl C, et al. Impact of body mass index on the efficacy of endocrine therapy in premenopausal patients with breast cancer: an analysis of the prospective ABCSG-12 trial. J Clin Oncol 2011;29:2653-9. [PubMed]
- Day R, National Surgical Adjuvant Breast and Bowel Projet P-1 study (NSABP-1). Quality of life and tamoxifen in a breast cancer prevention trial: a summary of findings from the NSABP P-1 study. National Surgical Adjuvant Breast and Bowel Project. Ann N Y Acad Sci 2001;949:143-50. [PubMed]
- Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1. J Clin Oncol 2010;28:641-7. [PubMed]
- Bordeleau L, Pritchard KI, Loprinzi CL, et al. Multicenter, randomized, cross-over clinical trial of venlafaxine versus gabapentin for the management of hot flashes in breast cancer survivors. J Clin Oncol 2010;28:5147-52. [PubMed]
- Loprinzi CL, Sloan J, Stearns V, et al. Newer antidepressants and gabapentin for hot flashes: an individual patient pooled analysis. J Clin Oncol 2009;27:2831-7. [PubMed]
- Fisher B, Costantino JP, Redmond CK, et al. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst 1994;86:527-37. [PubMed]
- Braithwaite RS, Chlebowski RT, Lau J, et al. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med 2003;18:937-47. [PubMed]
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998;90:1371-88. [PubMed]
- Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet 2002;360:817-24. [PubMed]
- Braems G, Denys H, De Wever O, et al. Use of tamoxifen before and during pregnancy. Oncologist 2011;16:1547-51. [PubMed]
- Ayoub JP, Valero V, Hortobagyi GN. Tamoxifen-induced female androgenetic alopecia in a patient with breast cancer. Ann Intern Med 1997;126:745-6. [PubMed]
- Puglisi F, Aprile G, Sobrero A. Tamoxifen-induced total alopecia. Ann Intern Med 2001;134:1154-5. [PubMed]
- Metindir J, Aslan S, Bilir G. Ovarian cyst formation in patients using tamoxifen for breast cancer. Jpn J Clin Oncol 2005;35:607-11. [PubMed]
- Beattie MS, Costantino JP, Cummings SR, et al. Endogenous sex hormones, breast cancer risk, and tamoxifen response: an ancillary study in the NSABP Breast Cancer Prevention Trial (P-1). J Natl Cancer Inst 2006;98:110-5. [PubMed]
- Sverrisdóttir A, Fornander T, Jacobsson H, et al. Bone mineral density among premenopausal women with early breast cancer in a randomized trial of adjuvant endocrine therapy. J Clin Oncol 2004;22:3694-9. [PubMed]
- Vehmanen L, Elomaa I, Blomqvist C, et al. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. J Clin Oncol 2006;24:675-80. [PubMed]
- Aft R. Protection of bone in premenopausal women with breast cancer: focus on zoledronic acid. Int J Womens Health 2012;4:569-76. [PubMed]
- Body JJ. Prevention and treatment of side-effects of systemic treatment: bone loss. Ann Oncol 2010;21 Suppl 7:vii180-5. [PubMed]
- Hadji P, Gnant M, Aapro M, et al. Dosing of zoledronic acid throughout the treatment continuum in breast cancer. Crit Rev Oncol Hematol 2011;79:175-88. [PubMed]
- Hojan K, Milecki P, Molińska-Glura M, et al. Effect of physical activity on bone strength and body composition in breast cancer premenopausal women during endocrine therapy. Eur J Phys Rehabil Med 2013;49:331-9. [PubMed]
- Paganini-Hill A, Clark LJ. Preliminary assessment of cognitive function in breast cancer patients treated with tamoxifen. Breast Cancer Res Treat 2000;64:165-76. [PubMed]
- Nystedt M, Berglund G, Bolund C, et al. Side effects of adjuvant endocrine treatment in premenopausal breast cancer patients: a prospective randomized study. J Clin Oncol 2003;21:1836-44. [PubMed]
- Colleoni M, Giobbie-Hurder A. Benefits and adverse effects of endocrine therapy. Ann Oncol 2010;21 Suppl 7:vii107-11. [PubMed]
- Berglund G, Nystedt M, Bolund C, et al. Effect of endocrine treatment on sexuality in premenopausal breast cancer patients: a prospective randomized study. J Clin Oncol 2001;19:2788-96. [PubMed]
- Ruddy KJ, Gelber S, Ginsburg ES, et al. Menopausal symptoms and fertility concerns in premenopausal breast cancer survivors: a comparison to age- and gravidity-matched controls. Menopause 2011;18:105-8. [PubMed]
- Christinat A, Pagani O. Fertility after breast cancer. Maturitas 2012;73:191-6. [PubMed]
- Davis AL, Klitus M, Mintzer DM. Chemotherapy-induced amenorrhea from adjuvant breast cancer treatment: the effect of the addition of taxanes. Clin Breast Cancer 2005;6:421-4. [PubMed]
- Azim HA, Kroman N, Paesmans M, et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: a multicenter retrospective study. J Clin Oncol 2013;31:73-9. [PubMed]
- Pagani O, Azim H Jr. Pregnancy after Breast Cancer: Myths and Facts. Breast Care (Basel) 2012;7:210-4. [PubMed]
- Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 2010;11:1135-41. [PubMed]
- Kumar NB, Allen K, Cantor A, et al. Weight gain associated with adjuvant tamoxifen therapy in stage I and II breast cancer: fact or artifact? Breast Cancer Res Treat 1997;44:135-43. [PubMed]
- Sestak I, Harvie M, Howell A, et al. Weight change associated with anastrozole and tamoxifen treatment in postmenopausal women with or at high risk of developing breast cancer. Breast Cancer Res Treat 2012;134:727-34. [PubMed]
- Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst 2007;99:272-82. [PubMed]
- Caan BJ, Kwan ML, Shu XO, et al. Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev 2012;21:1260-71. [PubMed]
- Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol 2002;20:1128-43. [PubMed]
- Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol 2007;25:2345-51. [PubMed]
- Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 2010;28:4120-8. [PubMed]
- Huiart L, Bouhnik AD, Rey D, et al. Early discontinuation of tamoxifen intake in younger women with breast cancer: is it time to rethink the way it is prescribed? Eur J Cancer 2012;48:1939-46. [PubMed]
- Carlson RW, Anderson BO, Burstein HJ, et al. Invasive breast cancer. J Natl Compr Canc Netw 2007;5:246-312. [PubMed]
- Cheung KL Winterbottom L, Owers R. Goserelin plus anastrozole as first-line endocrine threrapy for premenopausal women with oestrogen receptor (ER) positive advanced breast cancer (ABC). J Clin Oncol 2005;23:abstr 731.
- Forward DP, Cheung KL, Jackson L, et al. Clinical and endocrine data for goserelin plus anastrozole as second-line endocrine therapy for premenopausal advanced breast cancer. Br J Cancer 2004;90:590-4. [PubMed]
- Nishimura R, Anan K, Yamamoto Y, et al. Efficacy of goserelin plus anastrozole in premenopausal women with advanced or recurrent breast cancer refractory to an LH-RH analogue with tamoxifen: results of the JMTO BC08-01 phase II trial. Oncol Rep 2013;29:1707-13. [PubMed]
- Croxtall JD, McKeage K. Fulvestrant: a review of its use in the management of hormone receptor-positive metastatic breast cancer in postmenopausal women. Drugs 2011;71:363-80. [PubMed]
- Bartsch R, Bago-Horvath Z, Berghoff A, et al. Ovarian function suppression and fulvestrant as endocrine therapy in premenopausal women with metastatic breast cancer. Eur J Cancer 2012;48:1932-8. [PubMed]
- Cardoso F, Bedard PL, Winer EP, et al. International guidelines for management of metastatic breast cancer: combination vs sequential single-agent chemotherapy. J Natl Cancer Inst 2009;101:1174-81. [PubMed]


