The EMPIRICUS trial—the final nail in the coffin of empirical antifungal therapy in the intensive care unit?
Introduction
Invasive candidiasis (IC) includes primary candidemia, intra-abdominal or deep-seated tissue candidiasis and a combination of both, and is associated with a considerable morbidity and mortality in critically ill patients (1). In a recent point prevalence survey, the presence of Candida species was confirmed in 17% of infected culture-positive intensive care unit (ICU) patients (2). More than a dozen risk factors for IC have been identified such as major abdominal surgery, necrotizing pancreatitis, immunosuppression, use of broad-spectrum antibiotics, central vascular catheters, and Candida colonization (3). The latter is a preceding event in the natural history of IC. In particular, colonization at multiple body sites has been shown to be a strong predictor of subsequent IC episodes (4). In ICU patients the rate of colonization with Candida spp. is steadily increasing over time from 5–10% to 50–80%.
The overall mortality in ICU patients with IC is 30–40% (3) and may even approach 80% (5). Cohort studies indicate that timely administration of appropriate antifungal therapy and adequate source control are paramount for a favourable IC outcome (6). Consequently, earlier diagnosis using non-culture based techniques or prediction scores to identify patients at risk for IC in combination with pre-emptive or empiric antifungal treatment is attractive. The concept of empirical treatment for IC is employed in patients at-risk for IC and evidence of sepsis of unknown origin. Previous randomized controlled trials evaluating a prophylactic/pre-emptive (7-9) or empirical (10) antifungal treatment approach in ICU patients were not successful in demonstrating a reduction in overall mortality compared to placebo. It is noteworthy, that none of these trials incorporated Candida colonization status in the inclusion criteria as a risk assessment approach to identify patients at high risk for IC. Despite these disappointing results, current guidelines still recommend empirical treatment for suspected IC in the ICU (11), which may lead to significant over-treatment of ICU patients (12).
Study findings
In a recent multicentre, randomized, placebo-controlled trial (EMPIRICUS) published in the October issue of JAMA (13), Timsit et al. compared the outcome of a 14-day empirical treatment course of the echinocandin micafungin and placebo in 260 critically ill adult patients colonized with Candida spp. at one or more body sites outside the digestive tract. The investigators recruited subjects from 19 ICUs in France over a 3-year period, who were (I) mechanically ventilated for at least 5 days; (II) with at least 1 additional organ dysfunction; (III) with previous broad-spectrum antibacterial treatment for more than 4 days; (IV) with the presence of an arterial or central venous catheter; and (V) with new-onset ICU-acquired sepsis of unknown origin. Patients with neutropenia, previous stem cell or solid organ transplantation, immunosuppression and previous antifungal treatment were excluded. Twenty-eight-day survival free of proven invasive fungal infection was chosen as primary outcome.
Although many high-risk patients were recruited, as reflected by a median number of colonization sites of three, a considerable number of patients on hemofiltration and parenteral nutrition (33% and 26%, respectively), and a high median SAPS II score [48] at admission, it is noteworthy that patients with complicated abdominal surgery or necrotizing pancreatitis were clearly underrepresented. The primary endpoint, survival to day 28 free of invasive fungal infection, occurred in 68% and 60.2% of patients in the micafungin and placebo group (not significant), and various predefined subgroup analyses yielded similar results. Likewise, overall survival was not significantly different between the two groups. However, the incidence of proven invasive fungal infection during follow-up was significantly lower in the micafungin group [15 (12%) vs. 3 (3%), respectively, P=0.008]. There was no significant difference observed in other secondary endpoints including the course of β-D-glucan during follow-up. Pharmacokinetic analyses revealed a moderately decreased micafungin exposure (25%) compared with non-ICU patients.
Commentary
IC is an uncommon but severe complication in critically ill patients. Recent randomized controlled trials of prophylactic, pre-emptive or empiric antifungal treatment in high-risk ICU patients have failed to demonstrate a survival benefit (7-10). The current study results are important regarding two aspects. First, they confirm the lack of survival benefit of empirical antifungal treatment in critically ill patients, even when using a fungicidal echinocandin (as compared to fungistatic treatment with e.g., fluconazole). Second, it questions the benefit of repeated sampling for Candida colonization.
Lack of survival benefit
Although the current study demonstrated a reduced incidence of invasive fungal infections in the empirical treatment group, this did not translate into a survival benefit.
There are several possible explanations for these apparently discrepant findings. First, empirical antifungal treatment may obscure diagnosis of invasive Candida infections by e.g. decreasing sensitivity of blood cultures while it may not influence the development and outcome of invasive disease (14). Second, Candida colonization and infection may arise as one of several life-threatening complications in critically ill patients. Hence, Candida colonization and ultimately IC may serve as a marker of severity of the primary illness (15), and consequently the impact of empiric antifungal treatment may be limited. Third, critically ill patients as included in the current study are a heterogeneous population with a great variability in the risk of Candida colonization and more significantly of Candida infection. Retrospectively, micafungin treatment was successfully administered as empiric treatment in the minority of patients in the current study (12% of patients in the placebo group were diagnosed with IC), and hence the current study investigated a prophylactic/pre-emptive approach for IC in the majority of patients, which was not successful in previous trials (7,8). Overall, IC is a rather uncommon event in the general ICU population, and the number needed to prevent IC is high. Forth, micafungin exposure was reduced compared to non-ICU subjects. However, with a mean AUC of 78.2 mg*h/L in the current study, pharmacological target attainment [AUC0-24/MIC >3,000 (16)] for non-parapsilosis Candida spp. should have been achieved in the majority of patients, at least in the bloodstream. And although target attainment for C. parapsilosis would have been much lower in the current study [using a target of AUC0-24/MIC >285 (16)], the fact that not a single infection with C. parapsilosis was detected in the micafungin group argues against a major pharmacological issue with the chosen standard micafungin dose.
Candida colonization
Major risk factors for IC in ICU patients are common and in general have poor discriminative power. Currently available prediction scores or biomarkers have reasonably good negative predictive values, and may be used for antifungal stewardship purposes. However, their positive predictive value for invasive fungal disease in critically ill patients is rather disappointing. For example, β-D-glucan concentration does not only correlate with the incidence of IC but also with Candida colonization (17), and false-positive results have been reported in the presence of certain antibiotics or blood products (18). Consequently, current tests and prediction rules may lead to both significant overtreatment (almost 90% of patients in the placebo group did not develop IC) and missed opportunities in a heterogeneous ICU population with sepsis of unknown origin. In particular, the cost-effectiveness and usefulness of the Candida colonization status, a work-intensive strategy, for the management of ICU patients at risk for IC has not yet been demonstrated, and the current study clearly suggests that it should not be used on a routine basis. Future molecular tests that enable diagnosis of fungal bloodstream infections directly from whole blood may be more suited to trigger targeted antifungal therapy many days in advance of traditional culture-based methods without unnecessary treatment of patients at risk who will ultimately not progress to IC (19).
Outlook
What are the consequences of the negative findings of the EMPIRICUS trial (13)? First, the success of a one-size fits all approach of untargeted antifungal treatment in ICU patients at risk is doubtful (20), even when using fungicidal echinocandins. Second, the routine implementation of screening for Candida colonization in order to rule in patients who may benefit from empirical antifungal treatment is not recommended.
However, the role of empirical antifungal treatment in very high-risk patients presenting with ICU-acquired sepsis of unknown origin still remains to be determined. The target population for this approach may include patients on immunosuppressive treatment, with complicated gastrointestinal surgery, and the sickest patients (e.g., SOFA score >8), in particular in ICUs with an incidence of IC of >5–10% (11). In contrast to the EMPIRICUS study empiric antifungal treatment should be re-evaluated after 72–96 hours depending on results from blood cultures or intraoperative specimen and response to treatment (Figure 1). This approach limits unnecessary long treatment courses (96 hours vs. 14 days in the EMPIRICUS trial) and may reduce the emergence of fungal resistance before sensitive diagnostic tests are available, that reliably detect candidemia 24–96 hours before blood cultures turn positive (19).
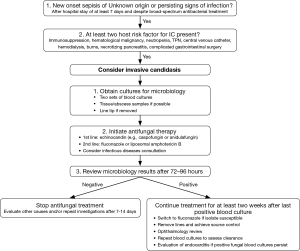
As a consequence of the EMPIRICUS trial, development of prediction score or diagnostic tests that have a high positive predictive value are mandatory before future randomized controlled trials of pre-emptive/empiric antifungal treatment should be conducted. The results from the EMPIRICUS trial serve as a cautionary note that more treatment does not necessarily translate into better outcomes. Future studies are required to decide if a pre-emptive/empiric approach to IC treatment should definitely be abandoned.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflict of interest to declare.
References
- León C, Ostrosky-Zeichner L, Schuster M. What's new in the clinical and diagnostic management of invasive candidiasis in critically ill patients. Intensive Care Med 2014;40:808-19. [Crossref] [PubMed]
- Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009;302:2323-9. [Crossref] [PubMed]
- Eggimann P, Bille J, Marchetti O. Diagnosis of invasive candidiasis in the ICU. Ann Intensive Care 2011;1:37. [Crossref] [PubMed]
- Pittet D, Monod M, Suter PM, et al. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg 1994;220:751-8. [Crossref] [PubMed]
- Marra AR, Camargo LF, Pignatari AC, et al. Nosocomial bloodstream infections in Brazilian hospitals: analysis of 2,563 cases from a prospective nationwide surveillance study. J Clin Microbiol 2011;49:1866-71. [Crossref] [PubMed]
- Kollef M, Micek S, Hampton N, et al. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis 2012;54:1739-46. [Crossref] [PubMed]
- Ostrosky-Zeichner L, Shoham S, Vazquez J, et al. MSG-01: A randomized, double-blind, placebo-controlled trial of caspofungin prophylaxis followed by preemptive therapy for invasive candidiasis in high-risk adults in the critical care setting. Clin Infect Dis 2014;58:1219-26. [Crossref] [PubMed]
- Knitsch W, Vincent JL, Utzolino S, et al. A randomized, placebo-controlled trial of preemptive antifungal therapy for the prevention of invasive candidiasis following gastrointestinal surgery for intra-abdominal infections. Clin Infect Dis 2015;61:1671-8. [PubMed]
- Pelz RK, Hendrix CW, Swoboda SM, et al. Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann Surg 2001;233:542-8. [Crossref] [PubMed]
- Schuster MG, Edwards JE Jr, Sobel JD, et al. Empirical fluconazole versus placebo for intensive care unit patients: a randomized trial. Ann Intern Med 2008;149:83-90. [Crossref] [PubMed]
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016;62:e1-50. [Crossref] [PubMed]
- Koo S, Bryar JM, Page JH, et al. Diagnostic performance of the (1-->3)-beta-D-glucan assay for invasive fungal disease. Clin Infect Dis 2009;49:1650-9. [Crossref] [PubMed]
- Timsit JF, Azoulay E, Schwebel C, et al. Empirical Micafungin Treatment and Survival Without Invasive Fungal Infection in Adults With ICU-Acquired Sepsis, Candida Colonization, and Multiple Organ Failure: The EMPIRICUS Randomized Clinical Trial. JAMA 2016;316:1555-64. [Crossref] [PubMed]
- Riedel S, Eisinger SW, Dam L, et al. Comparison of BD Bactec Plus Aerobic/F medium to VersaTREK Redox 1 blood culture medium for detection of Candida spp. in seeded blood culture specimens containing therapeutic levels of antifungal agents. J Clin Microbiol 2011;49:1524-9. [Crossref] [PubMed]
- Montravers P, Dupont H, Gauzit R, et al. Candida as a risk factor for mortality in peritonitis. Crit Care Med 2006;34:646-52. [Crossref] [PubMed]
- Andes D, Ambrose PG, Hammel JP, et al. Use of pharmacokinetic-pharmacodynamic analyses to optimize therapy with the systemic antifungal micafungin for invasive candidiasis or candidemia. Antimicrob Agents Chemother 2011;55:2113-21. [Crossref] [PubMed]
- Tissot F, Lamoth F, Hauser PM, et al. β-glucan antigenemia anticipates diagnosis of blood culture-negative intraabdominal candidiasis. Am J Respir Crit Care Med 2013;188:1100-9. [Crossref] [PubMed]
- Usami M, Ohata A, Horiuchi T, et al. Positive (1-->3)-beta-D-glucan in blood components and release of (1-->3)-beta-D-glucan from depth-type membrane filters for blood processing. Transfusion 2002;42:1189-95. [Crossref] [PubMed]
- Mylonakis E, Clancy CJ, Ostrosky-Zeichner L, et al. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clin Infect Dis 2015;60:892-9. [Crossref] [PubMed]
- Cortegiani A, Russotto V, Maggiore A, et al. Antifungal agents for preventing fungal infections in non-neutropenic critically ill patients. Cochrane Database Syst Rev 2016.CD004920. [PubMed]


