Characteristics and provision of care of patients with the acute respiratory distress syndrome: descriptive findings from the DACAPO cohort baseline and comparison with international findings
Introduction
The acute respiratory distress syndrome (ARDS) is a life-threatening condition characterized by either direct or indirect damage to the lung parenchyma causing critical hypoxemia and potentially hypercapnia and resulting in mechanical ventilation (1). In the year 2011 the Berlin-Definition revised and modified the American-European Consensus (AECC) diagnostic criteria of ARDS and acute lung injury (ALI) (2) that had been applied until that point. As a consequence the distinction between ARDS and ALI was removed and ARDS was classified in mild, moderate, and severe stages depending on the ratio of partial pressure arterial oxygen PaO2 and fraction of inspired oxygen FiO2 (PaO2/FIO2-ratio) (3). Even though a broad range of interventions to manage ARDS in intensive care was investigated and partly recommended in recent years, evidence that all these measures can substantially decrease mortality is still limited (4). Most of the evidence stems from high quality randomized controlled trials (RCTs) that include highly selected populations in strictly defined clinical settings. Data from such RCTs are urgently needed, but external validity is often limited. Therefore, transferring evidence from high quality RCTs into routine healthcare is problematic and effects found in RCTs will likely not be reproduced. Data collected in everyday life and/or everyday routine care are called real world data (RWD) (5) and are important to understand the complexities of ARDS outside the clinical trials setting. RWD obtained by observational study designs allow to investigate exposures, which are impossible to manipulate experimentally due to tight integration with the studied patients or ethical concerns. In this context some lifestyle risk factors such as cigarette smoke exposure or alcohol abuse (6), socio-demographic factors such as race and gender (7), and clinical comorbidity (8) have been investigated in rather small cohorts of ARDS patients. Larger cohorts allow to describe the clinical epidemiology of ARDS and to analyse associations between patient characteristics or care related factors and outcome measures. In this context, a closer look reveals a high degree of variability between different cohorts/samples. Some studies display major differences regarding to incidence and mortality (9). One reason for this variability might be that most European countries like the United Kingdom (10) or Germany (11) show considerable differences in the management of ARDS or other critical illnesses due the non-central clinical governance of health care provision.
Generally, there is a lack of descriptive socio-demographic and health care related data based on large and representative ARDS cohorts/samples. Therefore, we used data from the first 700 ARDS-patients enrolled in the DACAPO-Cohort [DACAPO: Surviving ARDS: The influence of quality of care and individual patient characteristics on health related quality of life and return to work in survivors of the ARDS] (12) for a descriptive analysis of socio-demographic and clinical characteristics as well as care-related factors of ARDS patients during intensive care unit (ICU) treatment. Our second aim was to compare the characteristics of the DACAPO cohort with findings of other large, multi-centric cohort studies.
Methods
Design and setting
Data were obtained from a large Germany-wide prospective cohort study [DACAPO study (12), ClinicalTrials.gov Identifier: NCT02637011]. Briefly, the DACAPO study investigates the influence of quality of care and individual patient characteristics on health-related quality of life (HRQoL) and return to work in survivors of ARDS. The data presented in this paper were collected once during ICU treatment, the design of the study is thus cross-sectional. In order to represent the entire spectrum of intensive health care service, ICUs with different medical specialization were invited all over Germany to participate in the DACAPO-study. Finally, 59 ICUs of 415 German hospitals with at least one department for intensive care (13) contributed eligible patients to the DACAPO cohort.
Eligibility and identification of cases
Inclusion criteria for patient enrolment were the presence of an ARDS according to the Berlin definition (3) and being 18 years of age or older. No exclusion criteria were applied.
ARDS was diagnosed by the medical team of the respective study site. In order to minimize sources of selection and information bias the responsible physicians and study nurses of the participating ICUs underwent a training regarding to the diagnostic criteria of ARDS Berlin definition, the assessment and the documentation of the medical and socio-demographic characteristics of interest.
Because of the consecutive inclusion of the participating ICUs, the period of patient recruitment varied between the study sites. Overall, 700 patients were enrolled in 59 study sites from September 2014 to January 2016. Data of these first 700 patients of the DACAPO-cohort were analysed for the purpose of this study.
Measures and data collection
We present descriptive data on the following variables of interest:
- General characteristics of participating study sites (e.g., level of care, university hospital) and their recruitment activities are reported;
- Socio-demographic characteristics of patients include sex, age, educational and professional level, current employment and living situation. This information was provided by patients, patients’ caregivers or legal guardians;
Clinical parameters comprise cause and severity of ARDS, diagnosis and therapeutic aim at admission as well as the presence of selected comorbidities. The Sequential Organ Failure Assessment score (SOFA) (14) and the Simplified Acute Physiology Score II (SAPS II) (15) were used to assess severity of illness at admission. Both scores were calculated according to the published algorithms (14,15). Additionally, the length of stay at ICU until death or discharge, the occurrence of critical events [hypoxemia (SpO2 <85% for at least 5 min), hypoglycemia (defined as blood glucose measurement <70 mg/dL), accidental extubation, re-intubation] was assessed. In the clinical intensive care setting, an arterial SpO2 <85%, corresponding to a PaO2 ≤50 mmHg with a time span of a few minutes, is accepted as a valuable marker for hypoxemia (16,17). The advocated measures in these situations are the control of artificial airways and ventilator function/modes/settings, the suction of endotracheal secretions, the acute use of open lung approaches, and/or acute imaging diagnostics (Chest X-ray, ultrasound). In addition the use of supportive measures [tracheotomy, NO-inhalation, extracorporeal membrane oxygenation (ECMO), prone positioning, neuro-muscular blockers] was recorded.
All clinical parameters were prospectively assessed and entered in electronic case report forms (eCRFs) by trained physicians or study personnel at individual study sites.
Statistics
Total scores were calculated only for patients without missing data on the item level. Descriptive statistics were calculated. Dichotomous or categorical parameters are presented in frequencies and percentages. Median (Md) and interquartile range (IQR) were calculated for continuous variables. All analyses were computed using IBM SPSS Statistics (version 21).
Systematic literature search
Additionally, we performed a systematic electronic search for observational studies of ARDS patients. We included only multi centre (more than one study site) studies with observational study design (cohort, case control, cross-sectional) and cohorts/samples larger than 200 patients in developed countries which were published from 01/01/2000 to 01/02/2016. To ensure high external validity of the reported findings, only cohorts/samples of ARDS patients without any exclusion criteria were selected. The retrieval of studies was performed in PubMed using the combined filter and Medical Subject Headings (MeSH) term: ((((“Respiratory Distress Syndrome, Adult”[MeSH Major Topic] AND (“2000/01/01”[PDAT]: “3000”[PDAT])) AND (“english”[Language] OR “german”[Language])) NOT animal[Filter]) AND “epidemiologic studies”[MeSH Terms]) NOT all child[Filter]. In a first step, the records were screened by two pairs of raters on the basis of title and abstract independently. Finally, the remaining records were appraised in detail by reading the full-text paper. Discrepancies were discussed between raters until consensus was achieved. All relevant characteristics of ARDS patients and medical care reported in the papers were extracted into evidence tables.
Results
Findings in the DACAPO-cohort
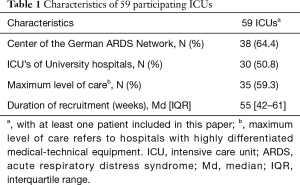
Thirty-eight (64%) of the 59 participating ICUs were centres of the German ARDS Network (18) and 30 ICUs of university hospitals participated (Table 1). As intended by the strategy which was applied to select the participating study sites, these comprised ICUs with different specializations (e.g., surgery/operative, anesthesia, internal medicine) and of various sizes and were heterogeneous regarding level of care.
Full table
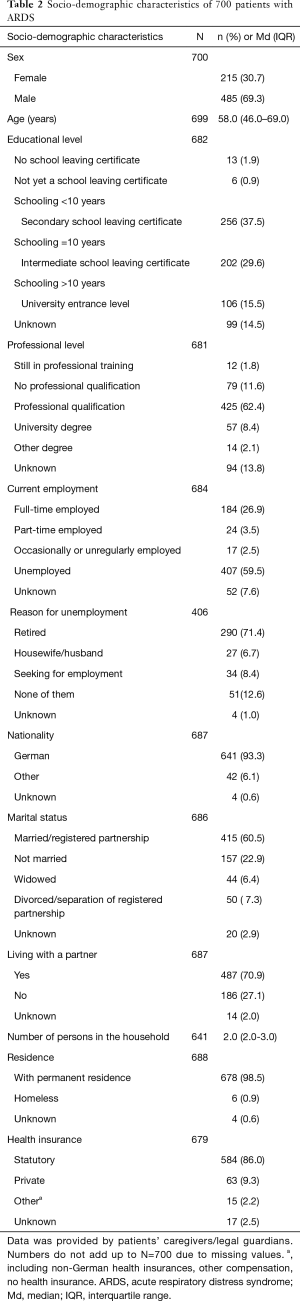
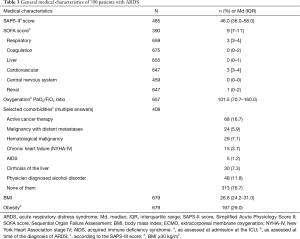
Socio-demographic characteristics are presented in Table 2. Two-thirds of the patients were male and median age was 58 (IQR =46.0–69.0) years. 16% of patients had a high schooling level qualifying for university entrance and 12% had no professional qualification. About a quarter of the sample (27%) were in full-time employment, the predominant reason for unemployment was retirement (71%). 61% were married/in a civil partnership and 71% lived with a partner. Important medical characteristics at ICU admission are displayed in Table 3. SAPS-II- (Md =46; IQR =36.0–58.0) and SOFA-scores (Md =9; IQR =7.0–11.0) represent a certain cohort of critically ill patients. Especially cardiovascular and renal dysfunctions led to elevated SOFA item scores. The most frequent comorbidities included cancer and alcohol disorder. About one third (29%) of patients were obese (body mass index ≥30 kg/m2).
Full table
Full table
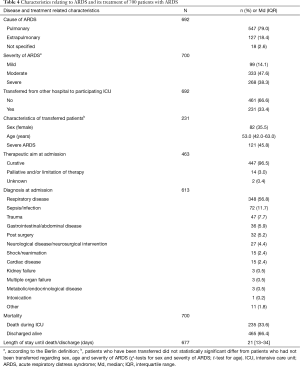
The main cause of ARDS was of pulmonary ‘direct’ origin (79%) (Table 4). The degree of severity of ARDS was unequally distributed in our sample, with nearly half of the cases classified as moderate, while ‘mild’ ARDS was only present in 14% of cases. At the time of diagnosis the median oxygenation level (PaO2/FiO2) was 101.5 (IQR =70.7–160.0). In one-third of patients (33%), ARDS had been diagnosed in referring hospital/ICU, before transfer to one of the participating ICUs. The percentage of ‘severe’ ARDS tended to be higher in the cohort of transferred patients (46% vs. 38% in the entire cohort). The leading diagnosis at ICU-admission was ‘respiratory disease’, while ‘trauma’ (8%), ‘gastrointestinal/abdominal disease’ (6%) or ‘post-surgery’ were rarely noted as main diagnoses. ICU mortality was 34%.
Full table
The occurrence of critical events and use of supportive measures are presented in Table 5. At least one critical event occurred in nearly half of the patients. Hypoxemic episodes were registered in 27% of patients, and 21% developed at least one hypoglycemic situation. A re-intubation was performed in 17%. The distribution of the use of supportive measures was as follows: less than half of ARDS patients received prone positioning (45%) and only a minority received neuromuscular blockers (11%). Overall, 425 out of 700 patients (61%) received at least one evidence-based rescue measure.

Full table
Findings in other large ARDS-cohorts
A total of 11 studies met the eligibility criteria applied to the studies found by the systematic literature search. Data extracted from these studies is shown in Table S1.

Full table
Not all cohorts/samples were independent. We identified an overlap of included patients in at least three studies (19-21).
A Taiwanese registry-based (National Health Insurance Research Database) study included more than 40,000 ARDS patients (22). Overall, the sample sizes of the studies detected by literature search ranged from 255 to 40,876. Three out of the 11 studies went beyond a national multi centre approach and included different international (23,24) or European (25) study sites.
Most of the large published cohorts/samples applied AECC diagnostic criteria. Only the most recent study (23) used the Berlin definition of ARDS.
Studies reported almost exclusively disease related factors. Socio-demographic and care related variables remained largely neglected. Exceptions were age (measures of central tendency ranged from 54 to 71 years) and sex (surplus of women in only one cohort). Mortality ranged from 33% (26) to 60% (24). SAPS II, APACHE (Acute Physiology and Chronic Health Evaluation) II &III and SOFA were commonly used measures for general morbidity. There was also no clear approach in reporting additional diseases. Some of the studies reported comorbidity others are limited to causes or risk factors of ARDS. Regardless of operationalization, sepsis was the most frequently recorded disease, closely followed by pneumonia.
Discussion
We aimed at providing a comprehensive description of 700 ARDS patients from the DACAPO cohort baseline, in order to add to the body of RWD on ARDS. We found that seventy percent of ARDS patients were male and that median age was 58 years. The majority of patients lived in a 2-person-household with partnership. These socio-demographic data suggest that the sample is likely reflective of the general population of this age in Germany, except for sex distribution (13). The latter observation is completely in accordance to other cohorts of ARDS patients listed in Table S1 and emphasizes the role of male sex as risk factor for heart and lung diseases in general and ARDS in particular. The finding of a high proportion of elderly patients is highly relevant, since old patients surviving a critical illness are at high risk for long-term physical and cognitive impairment (27) requiring prolonged care of the families and of the medical system (28). It must be determined in further health care studies, whether elderly patients receive adequate care after survival of ARDS.
In terms of severity, moderate ARDS was most frequently recorded, while mild ARDS was only reported in 14% of the patients. This observation is in line with the generally low oxygenation levels (PaO2/FiO2) of the DACAPO cohort and in contrast to the only cohort composed of ARDS patients diagnosed on the basis of the Berlin definition criteria (23). In this study the proportion of patients with mild ARDS was more than twofold higher (30%). Potentially, during routine clinical care the mild form of ARDS is under-diagnosed and overlooked even in university hospitals and/or hospitals providing a maximum level of care which participated in our study. Taking a closer look at the recorded concomitant illnesses, especially cancer and respiratory diseases are frequent in our patient sample. Because of a highly heterogeneous selection of concomitant diseases reported in the literature, a direct comparison of these results with findings of the existing scientific reports is very difficult. Some studies report causes or risk factors of ARDS while others present comorbidities with varying pooling. An obvious difference between the present data and results of other studies lies in the lower prevalence of sepsis (12%). One possible explanation for this could be the explicit recording of one main diagnosis at admission to ICU in the DACAPO study, whereas other studies recorded multiple diseases. Overall, pulmonary/respiratory diseases including pneumonia seem to be one of the most important cause or risk factors of ARDS, both in the large cohorts described in the literature and in the DACAPO-cohort. In addition the present data highlight the high prevalence of malignant cancer (active cancer therapy, malignancy with metastases, hematological malignancy) in patient with ARDS. Only one of the selected studies did address such an important comorbidity (25). Since the diagnosis ‘cancer’ influences intensive care markedly (29) and has certain consequences on the long-term care of surviving ARDS-patients, the high number of cancer patients in the cohort by Vincent et al. (25) and in the present cohort has an impact for the health care system and should be re-evaluated in large cohorts.
The cause of ARDS was assessed as pulmonary or ‘direct’ in 79% of patients. Although criticized by some authors (30), the differential assessment of the pathogenesis of ARDS (direct versus indirect) seems to be confirmed by recent biological findings which underline separate injurious pathways of pulmonary and non-pulmonary lung damage (31).
The high proportion of patients in which critical events (hypoglycemia, re-intubation, accidental extubation) (47%) occurred is surprising, considering that most participating study centres were ICUs of university hospitals or of hospitals with a maximum level of care. We found no ARDS specific data regarding the occurrence of critical events, but a prospective cohort study in an academic tertiary-care ICU revealed a proportion of patients with at least one critical adverse event of 19% (32). Furthermore the occurrence of such events was associated with longer duration of hospitalization. In a more recent study from the Mayo Clinic, USA, the effects of adverse events during ICU treatment were analyzed retrospectively in 828 acutely ill hospitalized patients with sepsis, shock, or pneumonia or undergoing high-risk surgery between 2001 and 2010 (32). All analyzed patients were at risk for or had developed ARDS. One adverse event increased the length of stay in the ICU by 2.4 (0.6–4.2) days. Beside the high proportion of patients with more severe ARDS, the latter finding could be an explanation for the extended length of ICU stay of the DACAPO-cohort (Md =21 days) compared to the cohort described by Bellani et al. (23) (Md =10 days). We conclude that the high occurrence of critical events in the management of ARDS patients is still a field in which patient safety initiatives, clinical care and medical education pathways need a better integration. Important elements for these initiatives include improving the reliability and standardization of processes of care, reducing unnecessary variation and complexity, and encouraging team working (33).
Finally, we recorded the use of evidence based supportive rescue measures in the treatment of ARDS-patients. Prone positioning and neuro-muscular blockers were only used in the minority of patients, although prone positioning is highly recommended in moderate-severe ARDS due to evidence from RCTs and meta analysis (34,35) . In addition a recent RCT demonstrated benefit of neuromuscular blockade in severe ARDS (36), but further studies are needed to investigate neuromuscular blockade as routine measure. ECMO was applied in 31% of patients, reflecting the increasing interest in and use of this technique in European countries. ECMO is recommended by a recent published consensus in critical hypoxemia despite optimized therapy (PaO2/FIO2 <70 for ≥3 hours) (37). In 2015 a survey was performed at German ARDS centres to determine the current treatment strategies in ARDS patients (11). It revealed that in accordance with our findings neuro-muscular blockers were periodically administered by 32%, and prone positioning was used by 60% of the centres. Thus, there is considerable scope for improving bedside implementation of evidence-based pathways in the management of ARDS in German ICUs.
The comparatively low mortality (34%) in our study is likely linked to the ARDS diagnosis criteria. While most studies applied the obsolete AECC criteria of ARDS which comprise only moderate and severe forms, the Berlin definition also includes mild ARDS. A comparison with the only cohort (23) that applied the Berlin definition criteria as we did reveals an almost perfect match of mortality rate. But inference has to be drawn considering the differences in the distributions of ARDS severity between these two cohorts.
Strengths of our study include the high number of enrolled ARDS patients without the application of any exclusion criteria form multiple sites all over Germany, the comprehensive assessment of socio-demographic and care-related data, the use of validated clinical severity scores, data collection by a standardized electronic data management system, and extensive quality assurance regarding missing and erroneous data. Our study also has some limitations: (I) we did not perform a registry-based study which is characterized by inclusion of all consecutively diagnosed ARDS cases. In Germany no institution or health-care regulation for an ARDS-registry exists, perhaps the present study might be a stimulation to found such a systematic registration; (II) there is a strong representation of hospitals belonging to the German ARDS network (specialized in the management of ARDS), potentially limiting external validity of our results; (III) generally, comparisons between data from older studies conducted in the ‘pre-Berlin-definition-era’ and data from our and other current studies using the Berlin-definition are problematic, since different diagnostic criteria are applied. Therefore, the investigated patient populations are not completely congruent and any conclusions must be drawn with caution.
Conclusions
Taking together, we conclude from our real world observational study that the characteristics of ARDS patients from the DACAPO cohort baseline are similar to other large ARDS-cohorts regarding age and sex distribution. The ICU-mortality is consistent with the mortality recorded by the only other study that applied the current Berlin definition of ARDS. Mild ARDS was underrepresented in our study, the reason being that it seems to be frequently overlooked in everyday routine care. Future studies should carefully consider this potential source of selection bias. Additionally the present study revealed a high occurrence of critical events during intensive care treatment. The implementation of evidence-based medicine (prone positioning and neuro-muscular blockers) seems to be still limited, pointing towards opportunities for improving current care. The lack of other large observational studies investigating socio-demographic characteristics of ARDS patients beyond age and sex is striking. We call for more research efforts to go beyond the description of medical characteristics and additionally focus on socio-demographic characteristics and life circumstances as well as comorbidities of patients suffering from ARDS, applying the Berlin definition in the first place.
Acknowledgements
We are indebted to all the intensivists and study nurses throughout Germany, who, with great commitment, recruited patients for the DACAPO study: Aachen, University Hospital RWTH Aachen, Department of Anesthesiology (Dr. Johannes Bickenbach, Dr. Thorben Beeker, Dr. Tobias Schürholz, Jessica Pezechk); Amberg, Klinikum Amberg, Department for Anaesthesiology (Dr. Jens Schloer); Augsburg, Klinikum Augsburg (Dr. Ulrich Jaschinski, Ilse Kreuzer); Bamberg, Sozialstiftung Bamberg Hospital, Department for Anaesthesiology (Dr. Oliver Kuckein); Berlin, Charité - University Medicine Berlin, Department of Anaesthesiology and Intensive Care Medicine (Dr. Steffen Weber-Carstens, Dr. Anton Goldmann, Dr. Stefan Angermair, Krista Stoycheva); Berlin, HELIOS Klinikum Berlin-Buch, Department of Intensive Care Medicine (Prof. Dr. Jörg Brederlau, Nadja Rieckehr, Gabriele Schreiber, Henriette Haennicke); Bielefeld, Ev. Krankenhaus Bielefeld. Department of Anesthesiology, Intensive Care Medicine, Emergency Medicine and Pain Therapy (Dr. Friedhelm Bach, Dr. Immo Gummelt, Dr. Silke Haas, Catharina Middeke, Dr. Ina Vedder, Marion Klaproth); Bochum, Ruhr University Bochum, Department of Anaesthesiology (Prof. Dr. Michael Adamzik, Dr. Jan Karlik, Dr. Stefan Martini, Luisa Robitzky); Bonn, University Hospital Bonn, Department of Anesthesiology and Intensive Care Medicine (Prof. Dr. Christian Putensen, Dr. Thomas Muders, Ute Lohmer); Bremen, Klinikum Bremen-Mitte, Department of Anesthesiology (Prof. Dr. Rolf Dembinski); Deggendorf, Medical Center, Department of Anaesthesiology and Intensive Care Medicine (Dr. Petra Schäffner, Dr. Petra Wulff-Werner); Dortmund, Klinikum Dortmund, Department of Critical Care Medicine (Elke Landsiedel-Mechenbier, Daniela Nickoleit-Bitzenberger, Ann-Kathrin Silber); Dresden, University Hospital Dresden Carl Gustav Carus, Department of Anesthesiology and Intensive Care Medicine (Prof. Dr. Maximilian Ragaller, Prof. Dr. Marcello Gama de Abreu, Alin Ulbricht, Linda Reisbach); Frankfurt am Main, University Hospital Frankfurt, Department of Anaethesiology, Intensive Care Medicine and Pain Therapy (Prof. Dr. Kai Zacharowski, Prof. Dr. Patrick Meybohm, Karin Pense, Gerhard Schwarzmann, Johannes Reske); Freiburg, University Medical Center Freiburg, Department of Anaesthesiology and Critical Care Medicine Freiburg (Prof. Dr. Alexander Hötzel, Dr. Johannes Kalbhenn); Freising, Klinikum Freising, Department of Anaesthesiology (Dr. Christoph Metz, Dr. Stefan Haschka); Göppingen, Klinik am Eichert, ALB FILS Kliniken, Department of Anaesthesiology and Intensive Care (Dr. Stefan Rauch); Göttingen, University Medical Center, Department of Anaesthesiology, Emergency and Intensive Care Medicine (Prof. Dr. Michael Quintel, Dr. Lars-Olav Harnisch, Dr. Sophie Baumann, Andrea Kernchen) ; Greifswald, University Medicine Greifswald, Department of Internal Medicine B (Dr. Sigrun Friesecke, Sebastian Maletzki); Hamburg, University Hospital Hamburg-Eppendorf, Department of Intensive Care Medicine, Center of Anesthesiology and Intensive Care Medicine (Prof. Dr. Stefan Kluge, Dr. Olaf Boenisch, Dr. Daniel Frings, Birgit Füllekrug, Dr. Nils Jahn, Dr. Knut Kampe, Grit Ringeis, Brigitte Singer, Dr. Robin Wüstenberg); Hannover, Hannover Medical School, Department of Anaesthesiology and Intensive Care Medicine (Dr. Jörg Ahrens, Dr. Heiner Ruschulte, Dr. Andre Gerdes, Dr. Matthias Groß); Hannover, Hannover Medical School, Department of Respiratory Medicine (Dr. Olaf Wiesner, Aleksandra Bayat-Graw); Heidelberg, University of Heidelberg, Department of Anaesthesiology (Dr. Thorsten Brenner, Dr. Felix Schmitt, Anna Lipinski) ; Herford, Klinikum Herford, Clinic for Anaesthesiology, Surgical Intensive Care Medicine, Emergency Care Medicine, Pain Management (Prof. Dr. Dietrich Henzler, Dr. Klaas Eickmeyer, Dr. Juliane Krebs, Iris Rodenberg); Homburg, Homburg University Medical Centre, Department of Anaesthesiology, Intensive Care and Pain Medicine (Dr. Heinrich Groesdonk, Kathrin Meiers, Karen Salm, Prof. Dr. Thomas Volk); Ibbenbüren, Ibbenbüren General Hospital, Division of Thoracic Surgery and Lung Support (Prof. Dr. Stefan Fischer, Basam Redwan); Immenstadt, Kempten-Oberallgaeu Hospitals, Clinic for Pneumology, Thoracic Oncology, Sleep- and Respiratory Critical Care (Dr. Martin Schmölz, Dr. Kathrin Schumann-Stoiber, Simone Eberl); Ingolstadt, Klinikum Ingolstadt, Department of Anaesthesiology and Critical Care Medicine (Prof. Dr. Gunther Lenz, Thomas von Wernitz-Keibel, Monika Zackel); Jena, Jena University Hospital, Deptartment of Anesthesiology and Intensive Care Therapy (Dr. Frank Bloos, Dr. Petra Bloos, Anke Braune, Anja Haucke, Almut Noack, Steffi Kolanos, Heike Kuhnsch, Karina Knuhr-Kohlberg); Kassel, Klinikum Kassel, Department of Anaesthesiology (Dr. Markus Gehling); Kempten, Klinikum Kempten-Oberallgäu gGmbH, Department for Anesthesia and Operative Intensive Care (Prof. Dr. Mathias Haller, Dr. Anne Sturm, Dr. Jannik Rossenbach); Kiel, University Medical Center Schleswig-Holstein, Campus Kiel, Department of Anesthesiology and Intensive Care Medicine (Dr. Dirk Schädler, Stefanie D‘Aria); Köln, Cologne-Merheim Hospital, Department of Pneumology and Critical Care Medicine (Dr. Christian Karagiannidis, Dr. Stephan Straßmann, Prof. Dr. Wolfram Windisch); Köln, University Hospital of Cologne, Department of Anaesthesiology and Intensive Care Medicine (Prof. Dr. Thorsten Annecke, Dr. Holger Herff); Langen, Asklepios Kliniken Langen-Seligenstadt, Department of Anesthesiology and Intensive Care Medicine (Dr. Michael Schütz); Leipzig, University of Leipzig, Department of Anesthesiology and Intensive Care Medicine (Dr. Sven Bercker, Hannah Reising, Mandy Dathe, Christian Schlegel) ; Ludwigsburg, Klinikum Ludwigsburg, Academic Teaching Hospital, University of Heidelberg, Department of Anaesthesiology (Katrin Lichy); Ludwigshafen, Klinikum Ludwigshafen, Department of Anesthesiology and Intensive Care Medicine (Prof. Dr. Wolfgang Zink, Dr. Jana Kötteritzsch); Mainz, University Medical Center Mainz, Department of Anaesthesiology (Dr. Marc Bodenstein, Susanne Mauff, Peter Straub); Magdeburg, Magdeburg University Medical Centre, Department of Anaesthesiology and Intensive Care Medicine (Dr. Christof Strang, Florian Prätsch, Prof. Dr. Thomas Hachenberg); Mannheim, University Medical Center Mannheim, Department of Anaesthesiology and Surgical Intensive Care Medicine (Dr. Thomas Kirschning, Dr. Thomas Friedrich, Dr. Dennis Mangold); Marburg, University Hospital, Department of Anaesthesiology (Dr. Caroline Rolfes, Tilo Koch); Mönchengladbach, Kliniken Maria-Hilf GmbH, Department of Cardiology (Dr. Hendrik Haake, Katrin Offermanns); München, Bogenhausen Hospital, Department of Anaesthesiology (Prof. Dr. Patrick Friederich, Dr. Florian Bingold); München, Klinikum Großhadern, Department of Anaesthesiology (Dr. Michael Irlbeck, Prof. Dr. Bernhard Zwissler); München, Klinikum Neuperlach, Städtisches Klinikum München GmbH, Department of Anesthesiology, Critical Care and Pain Medicine (Dr. Ines Kaufmann); München, Klinikum rechts der Isar, Department for Anaesthesiology of the Technical University of Munich (Dr. Ralph Bogdanski, Dr. Barbara Kapfer, Dr. Markus Heim, Dr. Günther Edenharter); Münster, University Hospital Münster, Department for Anaesthesiology, Intensive Care Medicine and Pain Therapy, (Prof. Dr. Björn Ellger, Daniela Bause); Neumarkt, Kliniken des Landkreises Neumarkt i.d.OPf, Department for Anaesthesiology and Intensive Care Medicine (Dr. Götz Gerresheim); Nürnberg, General Hospital Nuremberg, Paracelsus Medical University, Department of Emergency Medicine and Intensive Care (Dr. Dorothea Muschner, Prof. Dr. Michael Christ, Arnim Geise); Osnabrück, Marienhospital Osnabrück, Department of Anaesthesiology (Dr. Martin Beiderlinden, Dr. Thorsten Heuter); Passau, Klinikum Passau, Department for Anaesthesiology (Dr. Alexander Wipfel); Regensburg, Caritas Krankenhaus St. Josef, Department for Anaesthesiology (Dr. Werner Kargl, Dr. Marion Harth, Dr. Christian Englmeier); Regensburg, Regensburg University Hospital, Department of Anaesthesiology, Operative Intensive Care (Prof. Dr. Thomas Bein, Dr. Sebastian Blecha, Dr. Kathrin Thomann-Hackner, Marius Zeder); Stuttgart, Katharinenhospital, Department of Anesthesiology (Dr. Markus Stephan); Traunstein, Klinikum Traunstein, Department of Anaesthesiology (Dr. Martin Glaser); Tübingen, Tübingen University Hospital, Eberhard-Karls University Tübingen, Department of Anaesthesiology and Intensive Care Medicine (Dr. Helene Häberle); Ulm, Ulm University, Department of Anesthesiology (Prof. Dr. Hendrik Bracht, Christian Heer, Theresa Mast); Würzburg, University of Würzburg, Department of Anaesthesia and Critical Care (Dr. Markus Kredel, Dr. Ralf Müllenbach).
Further, we are grateful to previous members of the Regensburg DACAPO study team (medical documentation: Phillip Sebök, study physician: Kathrin Thomann-Hackner) to the members of the Advisory Board of the DACAPO-Study: Prof. Dr. Julika Loss, Prof. Dr. Bernhard Graf, Prof. Dr. Michael Leitzmann, Prof. Dr. Michael Pfeifer, Regensburg, Germany.
Funding: This work was supported by the German Ministry of Education and Research (Bundesministerium für Bildung und Forschung) (funding number: 01GY1340).
Footnote
Conflicts of Interest: S Kluge is member of the advisory board of Novalung and gives lectures in contract to Novalung. The other authors have no conflicts of interest to declare.
Ethical Statement: The study has been approved by the Ethics Committee of the University of Regensburg (file number 13-101-0262). Additionally approval was confirmed by the ethics committees of the participating study centres. Written informed consent was obtained from caregivers/legal guardians and additionally from those patients who survived ICU.
References
- Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 2012;122:2731-40. [Crossref] [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Tonelli AR, Zein J, Adams J, et al. Effects of interventions on survival in acute respiratory distress syndrome: an umbrella review of 159 published randomized trials and 29 meta-analyses. Intensive Care Med 2014;40:769-87. [Crossref] [PubMed]
- Price D, Brusselle G, Roche N, et al. Real-world research and its importance in respiratory medicine. Breathe (Sheffield, England) 2015;11:26-38. [Crossref] [PubMed]
- Moazed F, Calfee CS. Environmental risk factors for acute respiratory distress syndrome. Clinics in chest medicine 2014;35:625-37. [Crossref] [PubMed]
- Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979- 1996). Crit Care Med 2002;30:1679-85. [Crossref] [PubMed]
- Bein T, Hackner K, Zou T, et al. Socioeconomic status, severity of disease and level of family members' care in adult surgical intensive care patients: the prospective ECSSTASI study. Intensive Care Med 2012;38:612-9. [Crossref] [PubMed]
- Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome: incidence and mortality, has it changed? Curr Opin Crit Care 2014;20:3-9. [Crossref] [PubMed]
- Wise MP, Hart N, Frost PJ. ARDS outcomes: a marker of critical care quality in the UK? Thorax 2011;66:359. [Crossref] [PubMed]
- Kredel M, Bierbaum D, Lotz C, et al. Therapy of acute respiratory distress syndrome: Survey of German ARDS centers and scientific evidence. Der Anaesthesist 2015;64:277-85. [Crossref] [PubMed]
- Brandstetter S, Dodoo-Schittko F, Blecha S, et al. Influence of quality of care and individual patient characteristics on quality of life and return to work in survivors of the acute respiratory distress syndrome: protocol for a prospective, observational, multi-centre patient cohort study (DACAPO). BMC Health Serv Res 2015;15:563. [Crossref] [PubMed]
- Zensus 2011: Ergebnisse des Zensus / Sachsen-Anhalt, Statistisches Landesamt [Internet]. Sachsen-Anhalt / Statistisches Landesamt. [cited 2016]. Available online: https://ergebnisse.zensus2011.de/#
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. [Crossref] [PubMed]
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993;270:2957-63. [Crossref] [PubMed]
- Bein T, Grasso S, Moerer O, et al. The standard of care of patients with ARDS: ventilatory settings and rescue therapies for refractory hypoxemia. Intensive Care Med 2016;42:699-711. [Crossref] [PubMed]
- Khandelwal N, Hough CL, Bansal A, et al. Long-term survival in patients with severe acute respiratory distress syndrome and rescue therapies for refractory hypoxemia*. Crit Care Med 2014;42:1610-8. [Crossref] [PubMed]
- ARDS Netzwerk Deutschland. Available online: www.ardsnetwork.de
- Villar J, Blanco J, Anon JM, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 2011;37:1932-41. [Crossref] [PubMed]
- Villar J, Blanco J, del Campo R, et al. Assessment of PaO(2)/FiO(2) for stratification of patients with moderate and severe acute respiratory distress syndrome. BMJ open 2015;5:e006812. [Crossref] [PubMed]
- Villar J, Perez-Mendez L, Blanco J, et al. A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting--a prospective, multicenter validation study. Intensive Care Med 2013;39:583-92. [Crossref] [PubMed]
- Chen W, Chen YY, Tsai CF, et al. Incidence and Outcomes of Acute Respiratory Distress Syndrome: A Nationwide Registry-Based Study in Taiwan, 1997 to 2011. Medicine 2015;94:e1849. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Ferguson ND, Frutos-Vivar F, Esteban A, et al. Airway pressures, tidal volumes, and mortality in patients with acute respiratory distress syndrome. Crit Care Med 2005;33:21-30. [Crossref] [PubMed]
- Vincent JL, Sakr Y, Groeneveld J, et al. ARDS of early or late onset: does it make a difference? Chest 2010;137:81-7. [Crossref] [PubMed]
- Li G, Malinchoc M, Cartin-Ceba R, et al. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am J Respir Crit Care Med 2011;183:59-66. [Crossref] [PubMed]
- Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013;369:1306-16. [Crossref] [PubMed]
- Ehlenbach WJ, Larson EB, Curtis JR, et al. Physical Function and Disability After Acute Care and Critical Illness Hospitalizations in a Prospective Cohort of Older Adults. J Am Geriatr Soc 2015;63:2061-9. [Crossref] [PubMed]
- Kostakou E, Rovina N, Kyriakopoulou M, et al. Critically ill cancer patient in intensive care unit: issues that arise. J Crit Care 2014;29:817-22. [Crossref] [PubMed]
- Rocco PR, Pelosi P. Pulmonary and extrapulmonary acute respiratory distress syndrome: myth or reality? Curr Opin Crit Care 2008;14:50-5. [Crossref] [PubMed]
- Shaver CM, Bastarache JA. Clinical and biological heterogeneity in acute respiratory distress syndrome: direct versus indirect lung injury. Clin Chest Med 2014;35:639-53. [Crossref] [PubMed]
- Forster AJ, Kyeremanteng K, Hooper J, et al. The impact of adverse events in the intensive care unit on hospital mortality and length of stay. BMC Health Serv Res 2008;8:259. [Crossref] [PubMed]
- Ahmed AH, Thongprayoon C, Schenck LA, et al. Adverse in-hospital events are associated with increased in-hospital mortality and length of stay in patients with or at risk of acute respiratory distress syndrome. Mayo Clin Proc 2015;90:321-8. [Crossref] [PubMed]
- Park SY, Kim HJ, Yoo KH, et al. The efficacy and safety of prone positioning in adults patients with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. J Thorac Dis 2015;7:356-67. [PubMed]
- Guerin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Richard C, Argaud L, Blet A, et al. Extracorporeal life support for patients with acute respiratory distress syndrome: report of a Consensus Conference. Ann Intensive Care 2014;4:15. [Crossref] [PubMed]
- Hughes M, MacKirdy FN, Ross J, et al. Acute respiratory distress syndrome: an audit of incidence and outcome in Scottish intensive care units. Anaesthesia 2003;58:838-45. [Crossref] [PubMed]
- Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med 2004;30:51-61. [Crossref] [PubMed]
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685-93. [Crossref] [PubMed]


