C-reactive protein levels are raised in stable Chronic obstructive pulmonary disease patients independent of smoking behavior and biomass exposure
Introduction
Chronic obstructive pulmonary disease (COPD) is a major worldwide health problem with increasing prevalence and incidence. Although cigarette smoking is the most commonly encountered risk factor for COPD worldwide, some genetic and environmental risk factors are also well-identified in the disease pathogenesis. Still it is well-known that nonsmokers may also develop chronic airflow obstruction. Burning wood, animal dung, crop residues and coal in open fires or improper stoves may lead to serious indoor air pollution. The indoor air pollution resulting from biomass cooking or heating is an important risk factor for COPD especially in developing countries (1).
The chronic inflammation in COPD, orchestrated by multiple inflammatory cells and mediators in the airways and the lung tissues, is induced by inhalation of noxious gases and particulate matter. This persistent inflammatory response in the lung is also associated with a significant systemic inflammatory response yielding adverse clinical outcomes, so-called systemic effects of COPD (2). Although the origin of systemic inflammation present in COPD remains poorly understood and correlations in the regulation of inflammation in the pulmonary and systemic compartments are not well-documented yet, it is clearly established that some inflammatory markers are risen in systemic circulation (2,3). Of the blood-based biomarkers, C-reactive protein (CRP) has shown the greatest promise (4).
In COPD patients increased CRP levels are associated with poor lung function, reduced exercise capacity and worse quality of life as well as being a significant predictor of all-cause mortality (5-8). As well as COPD itself, smoking, which is the most commonly encountered risk factor for the disease is also responsible for rise in serum CRP levels (9). Though to our knowledge the effect of biomass exposure, potentially initiating inflammatory process in the lungs of COPD patients, on serum CRP levels has not been studied previously.
The growing awareness of COPD being a complex disease involving several organs with a clearly established low-grade systemic inflammation, biomarkers have been more focus of interest in clarifying the pathogenesis and progression of COPD as well as in designing new therapeutic targets for the disease (2).
Based on the current knowledge that COPD is a multicomponent systemic disease with elevated serum CRP levels and that smoking itself leads to rise in CRP levels, the present study is undertaken. With the study the authors aim to determine the relationship between serum CRP levels and well-known clinical parameters in COPD considering the impact of smoking behavior, biomass exposure and accompanying clinical entities, namely pulmonary hypertension, systemic hypertension and diabetes mellitus.
Materials and methods
Study population
This case-control study was conducted in Department of Chest Diseases Atatürk Chest Diseases and Surgery Training and Education Hospital, Ankara. Eighty-nine patients with COPD in clinically stable period were consecutively enrolled in the study between February 2008 and January 2009. Control group consisted of 60 age- and sex-matched healthy subjects with no history of COPD, confirmed by spirometric tests performed during medical examination prior to study entry.
COPD diagnosis for all patients included in the study was made by evaluation of pulmonary physicians in the tertiary hospital mentioned above. Patients with all stages of COPD were included if they had a post-bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) <70% after 400 mg of inhaled salbutamol. Patients enrolled in the study were all clinically stable based on having no exacerbation for the previous 2 months. Criteria of exacerbation were accepted as presence of one or more of three cardinal symptoms, including an increase or new onset of dyspnea, sputum production and sputum purulence (10). Staging of COPD was based on Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria using post-bronchodilator FEV1% predicted (1).
Exclusion criteria both for patients and control group were (I) history of asthma and/or FEV1 increase more than 12% and 200 mL after bronchodilation; (II) history or presence of tuberculosis, bronchiectasis and pulmonary embolus; (III) inflammatory diseases, such as malignancy, arthritis, connective tissue disorders or inflammatory bowel disease (11); (IV) use of statins or hormone replacement therapy (12,13); (V) ischaemic heart disease or heart failure, cerebrovascular or peripheric arterial disease (11); (VI) positive signs of active infection or recent trauma or surgery (11); and (VII) ongoing pulmonary rehabilitation programme (14).
Both written and verbal informed consent were obtained from each participant prior to study entry and the study protocol was approved by the ethics committee of the Atatürk Chest Diseases and Chest Surgery Training and Research Hospital.
Clinical assessment
Clinical assessment included detailed physical examination, routine blood tests and gathering information on current smoking status as well as smoking history, biomass exposure and accompanying diseases. Biomass exposure was based on the subjects’ statements and was assumed positive in adults who were born and lived in rural areas and routinely burn wood, animal dung, crop residues and coal for cooking and heating at least once or twice a week.
Pulmonary function tests and reversibility test was carried out in all subjects to detect airway obstruction and exclude reversible ones. Body-mass index (BMI) was calculated for all subjects. Oxygen saturation (SpO2) was detected with finger pulse oxymeter in both COPD patients and controls. Six minute walk test and assessment of dyspnea using modified Medical Research Council (MMRC) dyspnea scale were performed in COPD patients and BODE index scores were calculated accordingly.
Both COPD patients and controls underwent transthoracic echocardiograph, performed by a blind cardiologist, to record left ventricular ejection fraction and systolic pulmonary arterial pressure (sPAP). Pulmonary hypertension was considered positive as sPAP >35 mmHg at rest (15). Cardiac catheterization was not performed due to ethical reasons and since echocardiography is a valuable alternative noninvasive method for assessment of pulmonary hypertension (16).
Measurement of serum CRP levels
Venous blood samples were drawn from all subjects for CRP measurement at the same time of the day (9 am) in fasting state and samples were immediately centrifuged and obtained serum samples were immediately frozen at –80 °C until the time of analysis. High sensitive CRP measurements were performed by using CRPH reagent, Beckman Coulter (sensitivity ≤0.011 mg/dL) in conjunction with Beckman Coulter Immage® Immunohistochemistry System, CA, USA nephelometer.
Statistical analysis
Continuous variables were expressed as means ± SD and categorical variables were expressed as numbers and percentages. For comparison of the groups, independent Student’s t-test was applied for continuous variables if normally distributed or Mann Whitney-U or Kruskal Wallis test for data with non-normal distribution. The χ2-test was used for categorical variables. Correlations between serum CRP and other continuous variables were assessed using the Spearman’s correlation test. Correlations between serum CRP and categorical variables were assessed using Kendall’s tau-B.
Because of the non-normal distribution of CRP values, logarithmic transformation was used before performing a linear regression analysis. Stepwise regression analysis was employed with log-transformed CRP as a dependent variable to determine the best multivariate model predicting serum CRP levels.
All statistical tests were two-sided and a P-value <0.05 was considered statistically significant in all analyses. The analyses were assessed with the Statistical Package for Social Sciences (SPSS®) version 11.5 for Windows® system.
Results
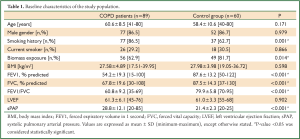
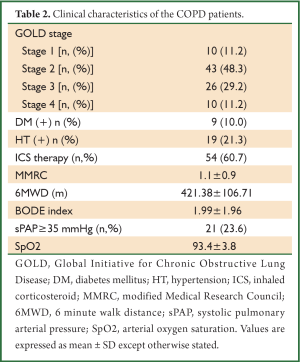
A total of 89 COPD patients (77 male) aged 41-80 years (mean 60.6 years) were recruited in the study. Also recruited into the study were 60 healthy subjects (52 males) from the general population, of similar age (mean 58.4 years) and location. Of the patients 29.2% were still smoking whereas 13.5% of them never smoked in their lives but had positive history for biomass exposure. The subjects included in the study with positive history of biomass exposure were exposed to high levels of indoor air pollution due to biomass cooking or heating everyday in their houses for at least 20 years. The baseline characteristics of the study groups are presented in Table 1. Most of the patients were in GOLD stages II and III and the rate of patients on inhaled corticosteroid (ICS) therapy in their regimen was 60.7%. Concomitant diabetes mellitus and systemic hypertension were encountered in COPD patients with incidences 10% and 21.3%, respectively. The clinical data of COPD patients is shown in Table 2.
Full Table
Full Table
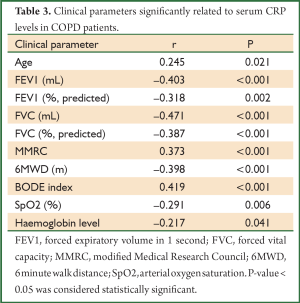
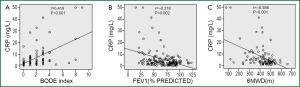
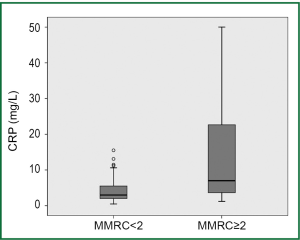
Serum CRP levels were significantly higher in COPD patients than in healthy subjects (7.22±9.84 vs. 3.14±2.27 mg/L, P=0.005). CRP levels were directly associated with age and inversely related to haemoglobin levels in COPD patients. Significant correlation was also found between CRP levels and the potential predictors of COPD severity including lung functions indices, oxygen saturation, dyspnea score, 6-minute walking distance (6MWD) and BODE scores (Table 3). CRP levels, on the other hand, were independent of the duration of the disease, BMI and sPAP levels in COPD patients. In COPD patients with low dyspnea score (MMRC <2) serum CRP levels were lower than those with high dyspnea score (MMRC ≥2) (4.59±4.69 vs. 14.33±15.38 mg/L, respectively; P<0.001). Linear relationship between FEV1% predicted, 6MWD and BODE scores is demonstrated in Figure 1 and the comparison of CRP levels between COPD patients with low and high levels of dyspnea in Figure 2. Serum CRP levels were not significantly different between COPD patients treated with ICS and those not treated (7.90±10.65 vs. 6.17±8.46 mg/L, respectively; P=0.504).
Full Table
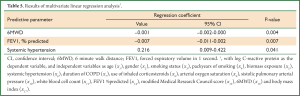
In order to explain serum CRP levels we planned stepwise regression analysis. To perform this regression model serum CRP levels were log-transformed to have normal distribution. Results of stepwise regression analysis model with log-transformed CRP as the dependent variable and independent variables as age (x1), gender (x2), smoking status (x3), packyears of smoking (x4), biomass exposure (x5), systemic hypertension (x6), duration of COPD (x7), use of inhaled corticosteroids (x8), SpO2 (x9), sPAP (x10), white blood cell count (x11), BODE index (x12) and stage of COPD (x13) is demonstrated in Table 4. According to these results multivariable model for predicting serum CRP levels contains BODE index scores and concomitant systemic hypertension. When components of BODE index (FEV1% predicted (x14), MMRC (x15), 6MWD (x16) and BMI (x17) were evaluated independently in the regression analysis instead of BODE index score (x12) and stage of COPD (x13), the best explanatory factors of log CRP levels were found as 6MWD, FEV1% predicted and systemic hypertension (Table 5).

Full Table
Full Table
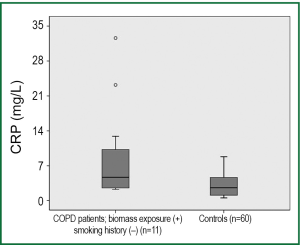
Seventy-seven of the COPD patients had positive smoking history whereas 12 patients had never smoked in their lives. On the other hand biomass exposure was positive in 56 COPD patients. Serum CRP levels did not differ significantly among COPD patients grouped according to smoking status and biomass exposure. Serum CRP levels were 8.96±9.59, 7.09±10.13 and 6.65±9.64 mg/L respectively in never-smoker (n=12), ex-smoker (n=51) and current smoker (n=26) COPD patients (P=0.316). Similarly, in COPD patients with positive history of biomass (n=56) serum CRP levels were 7.97±11.11 mg/L and in those with no history of biomass (n=33) it was 5.94±7.16 mg/L (P=0.244). Among those COPD patients who had never smoked in their lives 11 patients had positive history of biomass exposure together with no other potential risk factors for COPD. Accordingly, biomass exposure was the cause of COPD in this subgroup of patients. Serum CRP levels in the subgroup of COPD patients who never-smoked but have a positive history for biomass exposure were found higher than healthy controls (9.16±10.03 vs. 3.14±2.27 mg/L, respectively; P=0.028) (Figure 3).
With echocardiographic evaluation pulmonary hypertension [systolic pulmonary arterial pressure (sPAP) ≥35 mmHg] was encountered in 21 COPD patients. When compared to the rest of the COPD patients serum CRP levels were higher in COPD patients with pulmonary hypertension (11.86±13.38 vs. 5.78±8.05 mg/L, respectively; P=0.012).
Discussion
The main finding of the present study is that CRP levels are raised in stable COPD patients independent of smoking behavior and history of biomass exposure. Our results also demonstrate higher CRP levels were related to low FEV1% predicted, SpO2 and 6MWD and to high MMRC levels among the prognostic predictors of the disease, in concordance with the previous reports (5) and moreover indicate that serum CRP levels are most strongly related to BODE index and concomitant systemic hypertension.
With the growing awareness of COPD being a complex disease involving several organs with a clearly established low-grade systemic inflammation, biomarkers have been more focus of interest in clarifying the pathogenesis and progression of COPD as well as in designing new therapeutic targets for the disease (2). Elevated serum CRP levels indicating a low grade persistent systemic inflammation in COPD patients was first described in early 2000’s (17,18). Then positive relationship between CRP levels and important prognostic clinical variables in stable COPD patients was reported in 2006 by de Torres et al. (5). They published that CRP levels in stable COPD patients are associated with arterial oxygen tension, 6MWD, FEV1, FVC, inspiratory capacity/total lung capacity, GOLD stage of the disease and BODE index.
In the present study BODE index is the clinical parameter most strongly associated to serum CRP levels together with concomitant systemic hypertension in COPD patients. BODE index is a multidimensional grading system developed for monitoring the disease based on the arising knowledge of both pulmonary and extrapulmonary involvements are important for the clinical outcomes of COPD. This index is a good predictor of subsequent survival and is one of the best parameters for assessment of disease severity in COPD (19). This study also demonstrates that among the four components of the BODE index, exercise capacity, assessed by 6MWD, has the strongest association with serum CRP levels. These results indicate that systemic inflammation is related to the severity of the disease and physiological parameters should be considered in evaluating the COPD patients instead of focusing only on lung function indices.
Several cross-sectional and some prospective studies evaluating the relationship between blood pressure and CRP levels have reported a significant positive association inbetween. Although the papers have some methodological limitations, as a major cardiovascular risk factor, hypertension has been recently linked to inflammation (20). The findings of the present study suggest that coexistence of systemic hypertension and COPD exerts a higher degree of systemic inflammatory state which might have prognostic and therapeutic consequences if further investigated. On the other hand no such relationship was found for coexisting diabetes mellitus.
The airflow limitation in COPD is associated with abnormal inflammatory response of the lung to noxious particles or gases (1). Although in modernized sites of our country modern heating systems are used, in rural areas biomass heating and cooking is still traditionally important and is widely used. Cigarette smoking, on the other hand, is still the most commonly encountered risk factor (21). It is probable that such as in the case of smoking, biomass exposure also disturbs the cellular oxidant-antioxidant balance and initiates inflammatory process in the lungs of COPD patients. To our knowledge the effect of biomass exposure on serum CRP levels has not been studied previously. As mentioned above, in the present study no significant difference in serum CRP levels was detected between the COPD patients grouped according to smoking status and to history of biomass exposure. However in the subgroup of COPD patients with relevant biomass exposure who never smoked in their lives the serum CRP levels were significantly higher compared to healthy controls. With regard to these findings we confirm that elevated CRP levels in COPD patients is due to the ongoing inflammatory status and is independent of the smoking behaviour or biomass exposure.
Whether systemic inflammation is involved in development of pulmonary hypertension via pulmonary vascular remodeling in COPD patients is not well known yet. Although previously a significant linear relationship between serum CRP levels and sPAP levels was reported in a group of moderate to severe COPD patients (22), the role of inflammation in the pathogenesis of pulmonary hypertension in COPD patients is still controversial (23). Similarly in the present study COPD patients with pulmonary hypertension based on echocardiography had significantly higher serum CRP levels compared to those with normal sPAP levels. This finding is unsurprising since pulmonary hypertension is encountered in late stages of the disease and is associated with poor prognosis in COPD patients (24). High CRP levels have been shown to be associated with all-cause, cardiovascular, and cancer specific causes of mortality (8) thus reflect poor prognosis in COPD patients. On the contrary to those previous studies (22,25) the present study fails to reveal a relationship between sPAP values and serum CRP levels. However it is advisable to closely monitor the COPD patients with elevated CRP levels for evolving pulmonary hypertension with echocardiography.
Opposed to some previous reports declaring the use of inhaled corticosteroids decrease serum CRP levels (7,26) our data is unable to confirm any significant difference in serum CRP levels between COPD patients who were on inhaled corticosteroid therapy and those who were not. This is not surprising since the recent reports came along denying any reduction in systemic inflammation in COPD with the use of inhaled corticosteroids (27,28).
This study has some limitations. First pulmonary hypertension was assessed by echocardiographic evaluation which is accepted as a valuable alternative noninvasive method. Cardiac catheterization on the other hand was not performed due to ethical reasons. Secondly, CRP levels in some controls are above 3 mg/L which is the threshold considered of normality. So there is an increased serum levels in both COPD and controls. This is probably the effect of using nephelometry instead of an ELISA. Recently it was published that nephelometry may not be the best way to determine CRP serum levels in COPD since it has notable differences with ELISA (29).
Finally, we conclude that systemic inflammation is present in COPD patients and CRP is an important biomarker in COPD in means of reflecting disease severity and prognosis of patients. Serum CRP levels are risen independently of smoking status and biomass exposure in COPD patients reflecting that CRP rise was a result of the inflammatory nature of the disease itself. Systemic hypertension contributes to the degree of systemic inflammation, those COPD patients with concomitant systemic hypertension should more closely assessed for systemic effects of COPD and for worse prognosis. Further clinical trials must be held to investigate whether this condition has clinical implications in the follow-up and treatment of this subgroup of patients with concomitant hypertension, as well. Pulmonary hypertension is also related to a higher degree of inflammatory state in COPD patients which may be a result of more progressed disease. This issue should be assessed prospectively by measuring CRP over time and comparing the data with the hemodynamic measure of mean pulmonary artery pressure.
Acknowledgements
Funding: The authors would like to thank F. Keskin and D. İçen for their contribution in statistical analysis. The contents of this manuscript have been published in part as an abstract and poster at the 13th Annual Congress of the Turkish Thoracic Society, Istanbul 2010 and in European Respiratory Society Annual Congress, Vienna 2012.
Disclosure: The authors declare no conflict of interest.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy For The Diagnosis, Management And Prevention Of Chronic Obstructive Pulmonary Disease 2006.
- Agustí A. Systemic effects of chronic obstructive pulmonary disease: what we know and what we don’t know (but should). Proc Am Thorac Soc 2007;4:522-5. [PubMed]
- Wouters EF. Local and systemic inflammation in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2:26-33. [PubMed]
- Sin DD, Man SF. Biomarkers in COPD: are we there yet? Chest 2008;133:1296-8. [PubMed]
- de Torres JP, Cordoba-Lanus E, López-Aguilar C, et al. C-reactive protein levels and clinically important predictive outcomes in stable COPD patients. Eur Respir J 2006;27:902-7. [PubMed]
- Broekhuizen R, Wouters EF, Creutzberg EC, et al. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax 2006;61:17-22. [PubMed]
- Pinto-Plata VM, Müllerova H, Toso JF, et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax 2006;61:23-8. [PubMed]
- Man SF, Connett JE, Anthonisen NR, et al. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax 2006;61:849-53. [PubMed]
- Danesh J, Muir J, Wong YK, et al. Risk factors for coronary heart disease and acute-phase proteins. A population-based study. Eur Heart J 1999;20:954-9. [PubMed]
- Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106:196-204. [PubMed]
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003;111:1805-12. [PubMed]
- Albert MA, Danielson E, Rifai N, et al. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001;286:64-70. [PubMed]
- Ridker PM, Hennekens CH, Rifai N, et al. Hormone replacement therapy and increased plasma concentration of C-reactive protein. Circulation 1999;100:713-6. [PubMed]
- Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology 2002;13:561-8. [PubMed]
- Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004;43:40S-47S. [PubMed]
- Burgess MI, Mogulkoc N, Bright-Thomas RJ, et al. Comparison of echocardiographic markers of right ventricular function in determining prognosis in chronic pulmonary disease. J Am Soc Echocardiogr 2002;15:633-9. [PubMed]
- Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003;107:1514-9. [PubMed]
- Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and markers of inflammation: data from the Third National Health and Nutrition Examination. Am J Med 2003;114:758-62. [PubMed]
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005-12. [PubMed]
- Virdis A, Ghiadoni L, Plantinga Y, et al. C-reactive protein and hypertension: is there a causal relationship? Curr Pharm Des 2007;13:1693-8. [PubMed]
- Kocabas A, Hancioglu A, Turkyilmaz S, et al. Prevalance of COPD in Adana, Turkey (BOLD-Turkey Study). Proc Ame Thorac Soc 2006;3:A543.
- Joppa P, Petrasova D, Stancak B, et al. Systemic inflammation in patients with COPD and pulmonary hypertension. Chest 2006;130:326-33. [PubMed]
- Sin DD, Man SF. Is systemic inflammation responsible for pulmonary hypertension in COPD? Chest 2006;130:310-2. [PubMed]
- Weitzenblum E, Hirth C, Ducolone A, et al. Prognostic value of pulmonary artery pressure in chronic obstructive pulmonary disease. Thorax 1981;36:752-8. [PubMed]
- Kwon YS, Chi SY, Shin HJ, et al. Plasma C-reactive protein and endothelin-1 level in patients with chronic obstructive pulmonary disease and pulmonary hypertension. J Korean Med Sci 2010;25:1487-91. [PubMed]
- Sin DD, Lacy P, York E, et al. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:760-5. [PubMed]
- Sin DD, Man SF, Marciniuk DD, et al. The effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:1207-14. [PubMed]
- Miniati M, Monti S, Bottai M, et al. Prognostic value of C-reactive protein in chronic obstructive pulmonary disease. Intern Emerg Med 2011;6:423-30. [PubMed]
- López-Campos JL, Arellano E, Calero C, et al. Determination of inflammatory biomarkers in patients with COPD: a comparison of different assays. BMC Med Res Methodol 2012;12:40. [PubMed]


