Understanding local performance data for EBUS-TBNA: insights from an unselected case series at a high volume UK center
Introduction
The advent of convex-probe endobronchial ultrasound transbronchial aspiration (EBUS-TBNA) in 2005 has been a major development in respiratory medicine. It has allowed cytological diagnosis to be performed simultaneously with real-time imaging and enabled access to a wide range of mediastinal and hilar lymph node stations and peribronchial masses which previously would have required more invasive methods of diagnosis, such as mediastinoscopy, or would have been left to radiological follow up. In the past 10 years, numerous studies have been published supporting the high diagnostic accuracy of EBUS-TBNA in a range of settings: the staging of lung cancer, the diagnosis of cancer, including metastases from extrathoracic malignancies, and benign diseases of the mediastinum (1). The safety and cost effectiveness have been established over other methods of staging such as mediastinoscopy and numerous guidelines including the National Institute for Health and Care Excellence (NICE) (2), European Society of Thoracic Surgeons (ESTS) (3) and American College of Chest Physicians (ACCP) (4) now recommend EBUS-TBNA as first line modality for staging in lung cancer.
In the past two years, there has been a growth in the provision of EBUS services across the UK (5) and it is likely that this expansion will continue. This underpins the need for centers to evaluate their practice to reduce variation and ensure that standards are met. In 2014, the British Thoracic Society Quality Statement (6) published standards for sensitivity for EBUS-TBNA in the staging of lung cancer. These should however be considered as minimum standards only; Evison et al. have recently highlighted (7) the need to evaluate the performance of EBUS in staging in the context of disease prevalence which has an important influence on both sensitivity and negative predictive value (NPV).
The objective of this article is to review the diagnostic performance of EBUS-TBNA on an unselected large cohort of patients who underwent the procedure in our institution in the past 3 years and to compare against published standards as well as existing literature. We provide some of the insights that we have gained that may help avoid some common pitfalls related to the technical aspects of the procedure as well as data collection and analysis, in order to optimize the accuracy and interpretation of local performance data in EBUS-TBNA.
Methods
An EBUS service was set up at our institution in 2008. Since then the Trust has been performing over 250 cases annually. Our Trust provides the service for a large network of National Health Service trusts in the North East of England, although over time a number of other centers in the region have introduced their own service.
A database of all patients referred for EBUS-TBNA is kept. All patients who underwent the examination from January 2013 to December 2015 were included in the retrospective analysis. The decision to refer patients for EBUS-TBNA was at the discretion of the primary physician treating the patient.
EBUS was performed using Olympus BF-UC260FW and incremental doses of fentanyl and midazolam for conscious sedation. EBUS-TBNA was performed on pre-planned targeted areas such as lymph nodes which were radiologically enlarged, PET-positive or >0.5 cm in diameter on EBUS, and/or peribronchial masses depending on the indication of the EBUS. The number of lymph node stations sampled and number of aspirates per node were at the discretion of the operator. Targeted lymph nodes were punctured with either the 21 or 22 gauge EBUS-guided TBNA needles (NA-201SX-4021, NA-201SX-4022, Olympus Medical Systems), also at the discretion of the main operator. EBUS was performed in the absence of rapid on-site evaluation (ROSE). The aspirated specimens from each lymph node station were flushed into vials containing CytoLyt® (methanol-water solution) (8). For patients with IMHL, one pass was routinely submitted for acid-fast bacilli smear and TB culture. Immunohistochemistry was performed as required; however flow cytometry was not available. The procedures were performed by four experienced consultants.
Consecutive cases were reviewed and the following data recorded: age, gender, number of lymph node stations sampled, the number of passes per lymph node station, the pathological results of EBUS-TBNA, the pathological and microbiological results of any subsequent relevant sampling and the outcome of clinico-radiological follow-up up to August 2016. This enabled a minimum of 6 months’ follow-up for all patients but a much longer period of surveillance in the majority of cases. The CT scan and PET-CT scan reports, where applicable, were reviewed to confirm the indications of EBUS-TBNA. Based on the EBUS-TBNA results, subsequent sampling results and the outcome of clinico-radiological follow-up, a final diagnosis of carcinoma, sarcoidosis, TB, lymphoma, or reactive lymphadenopathy was made. A diagnosis of reactive lymphadenopathy was made only if all pathological sampling and a minimum of 6 months’ follow-up failed to provide an alternative diagnosis, and the referring physician did not consider the patient to have another diagnosis.
For assessing EBUS-TBNA performance, patients were analysed in three subgroups based on the indication for the EBUS-TBNA: to examine its utility in investigation of IMHL, its performance in the staging of suspected or confirmed non-small cell lung cancer (NSCLC) and for its ability to make a tissue diagnosis in suspected thoracic or extrathoracic cancer.
For the analysis of patients with radiological evidence of IMHL, patients who had concurrent active extrathoracic cancer or who had conglomerate invasive looking nodes suggestive of cancer were excluded.
For patients subjected to EBUS-TBNA for staging, accuracy of EBUS-TBNA was measured by its ability to determine the true N2 stage in suspected lung cancer. Thus, for example, patients who had N2 disease in nodal stations not accessible to EBUS-TBNA were labelled as false negatives. Patients were also further grouped according to the ACCP classifications (A-D) of intrathoracic staging based on the index staging CT report (Table 1) (4).

Full table
Statistical analyses were performed using a commercially available software programme (Medcalc version 16.8.4). Demographic and clinical characteristics of the study population were summarized using mean, standard deviation, median, range, or counts and percentages, depending on their type and distribution.
Sensitivity, accuracy and NPV were calculated using the standard definitions and expressed at the 95% binomial confidence interval. Specificity of EBUS-TBNA samples was assumed to be 100%.
Results
A total of 1,656 lymph nodes and 138 peribronchial/peritracheal masses were sampled in 940 patients over the 36-month study period. The patients’ median age was 70 years (range, 39–93 years). The male: female ratio was 1:1. Forty-seven percent of patients referred for EBUS-TBNA were from external NHS Trusts.
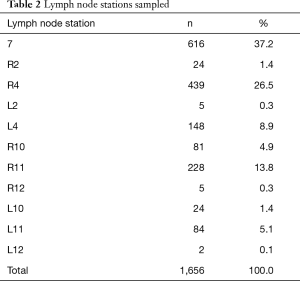
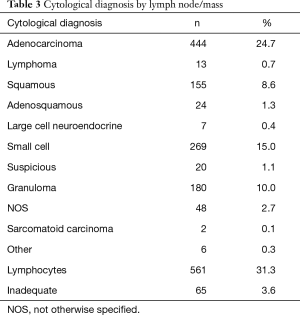
The lymph node stations sampled are shown in Table 2. The size in short axis was recorded in 1,442 lymph nodes and masses. The mean size of lymph nodes and masses in short axis were 1.7±0.8 and 2.7±0.9 cm respectively. The median number of passes per lymph node station or mass was 2 (range, 1–6). The overall cytological diagnosis by lymph node/mass is displayed in Table 3.
Full table
Full table
IMHL
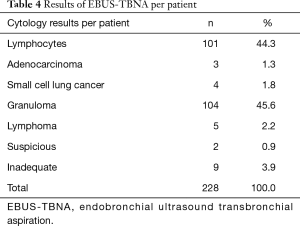
Two hundred and twenty-eighty patients were referred for the investigation of isolated mediastinal and/or lymphadenopathy in the absence of any active extrathoracic cancer. The mean age was 60±13 years. The male:female ratio was 1:1. The results of EBUS-TBNA per patient are displayed in Table 4.
Full table
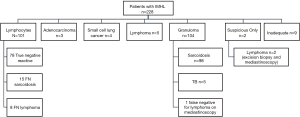
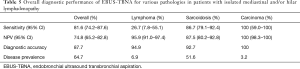
The outcome of the EBUS-TBNA findings in the 228 patients is displayed in Figure 1. Out of 101 patients in whom only lymphocytes were found, 16 proceeded to mediastinoscopy and 2 underwent alternative modalities of biopsy under the discretion of the treating physician. In 15 patients, a pathological diagnosis was made (6 with lymphoma, 9 with sarcoidosis). The remaining 83 patients underwent clinical-radiological follow up. Out of these, 6 were felt to be in keeping with sarcoidosis and 2 with lymphoma. The overall diagnostic performance of EBUS-TBNA for various pathologies in the subgroup of patients with IMHL is tabulated in Table 5. The prevalence of reactive lymphadenopathy was 34%. Based on the reports of the CT scans performed before EBUS-TBNA, it was estimated that the proportion of patients with Stage 1 sarcoidosis was 89.8% (n=88) and with Stage 2 were 10.2% (n=10).
Full table
Staging of lung cancer
A total of 294 patients underwent EBUS-TBNA for mediastinal staging of suspected lung cancer. Of 294, 291 underwent combined mediastinal staging and diagnosis, and 3 patients were known to have proven NSCLC and underwent EBUS-TBNA for staging purposes only. The latter were all external referrals from different Trusts. The mean age was 72±12 years. The male to female ratio was 1:1.
To assess the accuracy of EBUS-TBNA for predicting N2 disease in NSCLC, the following patients were included in the analysis: patients confirmed to have NSCLC on EBUS-TBNA or other modalities of sampling including mediastinoscopy or lymph node resection during surgery, as well as patients suspected to have and/or treated as NSCLC and who were followed up over at least 6 months with serial imaging. Patients subjected for staging had enlarged (>10 mm) mediastinal lymph nodes on CT and/or PET-positive mediastinal lymph nodes and/or had a central tumour and/or suspicion of N1 disease. Patients excluded from the analysis were those with ACCP Category A where staging was not required and the purpose of EBUS was diagnosis only, those proven on EBUS-TBNA to have small cell lung cancer (n=2) and those whose initial mass and lymph nodes significantly regressed on subsequent imaging suggesting a benign aetiology (n=67). The final subset included 225 patients of which 174 (77.3%) belonged to ACCP Category B and 51 (22.7%) from ACCP Category C. The mean size of node was 1.5±0.8 cm. The median number of lymph node stations sampled was 1.5 (range 1–4).
Out of 114 patients with lymphocytes only in mediastinal nodes, 68 proceeded to mediastinoscopy +/− thoracotomy and systematic lymph node sampling, 2 to repeat EBUS-TBNA, whilst 44 underwent clinico-radiological follow up with no additional pathological sampling due to various patient related factors, including non-operability and poor functional status. Mediastinoscopy identified further 7 (6.1%) patients with N2 disease, and thoracotomy identified further 2 (1.8%) patients with N2 disease in stations 5 and 8, both inaccessible to EBUS-TBNA and not detected on mediastinoscopy. The 2 patients who underwent repeat EBUS were staged as N2 on repeat testing. In 10 out the remaining 44 cases (22.7%), there was radiographic evidence of mediastinal progression. Although pathological confirmation was not performed in this group, these were assumed to be false negative.
The sensitivity for N2 staging was thus 83.7% (95% CI: 76.2–89.6%) with a NPV of 81.6% (95% CI: 73.2–88.2%) and a diagnostic accuracy of 87.8%. The prevalence of N2 disease was 58%.
In the subgroup of patients who proceeded to surgical sampling, the sensitivity was higher at 91% (95% CI: 88.4–95.4%) and a NPV of 84.3% (95% CI: 73.6–91.9%), with the N2/N3 disease prevalence of 67.4%.
Diagnosis of cancer
A total of 1,243 nodes and 138 masses were sampled to establish a tissue diagnosis in 707 patients suspected to have cancer. Patients with isolated mediastinal adenopathy in absence of any active thoracic or extrathoracic cancer were excluded from this group analysis and evaluated separately. The mean age was 71±14 years. The male to female ratio was 1:1.
Four hundred and sixteen (58.9%) patients were deemed to have radiological evidence of inoperable cancer with large volume conglomerate nodal disease and/or presence of intrathoracic or distant metastases. In 291 patients, EBUS-TBNA was performed for both diagnosis and staging purposes in one sitting. The mean size of node/mass was 1.8±0.9 cm. The results of EBUS-TBNA per patient are displayed on Figure 2. The outcome of the patients is displayed on Figure 3.
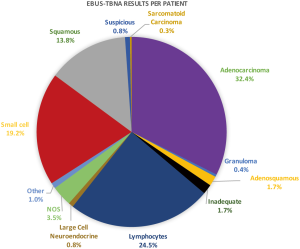
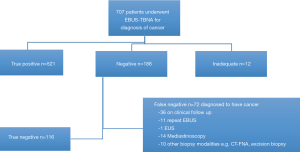
The sensitivity of EBUS-TBNA to make a tissue diagnosis of thoracic or extrathoracic cancer was 88% (95% CI: 85.1–90.5%) and a NPV of 62% (95% CI: 54.7–69.0%). The disease prevalence was 83.6%.
Additionally, 27 patients with known active extrathoracic cancer underwent EBUS-TBNA for sampling mediastinal nodes/mass. The performance of EBUS-TBNA in the subgroup was higher with a sensitivity of 95.7% (95% CI: 78.1–99.9%) and a NPV of 80% (95% CI: 28.4–99.5%). The primaries included as follows: breast n=12 (44.4%), genitourinary n=9 (33.3%), head and neck n=4 (4.9%), colorectal n=2 (7.4%).
Discussion
Our study evaluated the performance of EBUS-TBNA in three main categories—in investigation of IMHL, in mediastinal staging (i.e., N2/N3 disease) of patients with suspected or confirmed NSCLC, and in the ability of EBUS-TBNA to provide a pathological diagnosis in patients suspected to have thoracic and extrathoracic cancer.
IMHL
This study provides data on the largest retrospective series of patients referred for the investigation of IMHL in the absence of any active extrathoracic malignancy. This is a common indication for patients referred for EBUS-TBNA and constituted about 25% of our patients. Common causes include sarcoidosis, tuberculosis, lymphoma, and metastatic carcinoma. Reactive lymphadenopathy can be associated with a number of conditions, including pulmonary fibrosis, pulmonary infections and heart failure.
The study demonstrates that EBUS-TBNA is a highly effective diagnostic modality for this indication, making a correct diagnosis in 88%, with an overall sensitivity of 81.6% (95% CI: 74.2–87.6%) and a NPV of 74.8% (95% CI: 65.2–82.8%), with a disease prevalence of 64.7%. This compares favourably with both retrospective and prospective (the REMEDY trial) case series examining the role of EBUS-TBNA in IMHL (9,10). The REMEDY Trial was performed in a highly selected cohort of patients with a high pre-test probability of pathological disease, with all EBUS-TBNA negative cases subjected to mediastinoscopy. The very low prevalence of reactive lymphadenopathy (5%) in that trial partly explains the lower NPV of 40% compared to our study group. This reflects the need to report the NPV in conjunction with the prevalence of pathological disease, with a higher disease prevalence resulting in a lower NPV.
In our study, the diagnosis of reactive IMHL was made after negative EBUS sampling and, in the majority of cases, clinical/radiological follow-up, based on the index of suspicion and at the discretion of the treating physician. Only a minority of patients underwent additional sampling, raising the possibility of inaccurate categorization. However, in the majority of patients, follow-up was for a longer than 6 months and further sampling was not required in those patients.
Sarcoidosis
A high diagnostic yield for EBUS-TBNA was found in a recent paper by Agarwal et al. (11) who performed a meta-analysis of studies of EBUS-TBNA on highly selected patients with high suspicion of sarcoidosis (prevalence of around 80%), leading to risk of overestimation of test performance of EBUS-TBNA. This may not reflect real life data or be a true representation of the typical cohort of patients referred to an EBUS service.
However, another large meta-analysis (12) of unselected patients with intrathoracic lymphadenopathy showed a pooled diagnostic yield of 79% (SD 24%) and a sensitivity of 84% (95% CI: 79–88%), despite an overall median prevalence of only 15%. Similarly, a recent retrospective series on a cohort of 100 unselected patients with IMHL showed a sensitivity of 80% for diagnosing sarcoidosis with a disease prevalence of 20% in the study cohort (9).
In the current study, EBUS-TBNA demonstrated a high sensitivity of 86.7% (95% CI: 79.1–92.4%) and diagnostic yields of 86.7% (98/113), which compare favourably with the current literature.
Our data also compares well with studies of mediastinoscopy (13,14) on patients with IMHL which report a sensitivity range of 74–97% for diagnosing sarcoidosis, thus demonstrating the utility of EBUS-TBNA as a first line test for a clinically unselected cohort of patients referred for investigation of IMHL.
Although our current data shows excellent performance characteristics in sarcoidosis, caution needs to be exercised before interpreting data. Approximately 90% of our patient population with sarcoidosis had radiographic stage I sarcoidosis. Evidence suggests that the yield of both conventional and EBUS-TBNA (15,16) is higher in stage I than in stage II sarcoidosis, probably due to a higher density of granulomas in lymph nodes of stage I patients. This may thus in part explain the high yield in the current study group.
The impact of the processing method for specimens retrieved with EBUS-TBNA is difficult to ascertain but may be of relevance. A study by Schwartz et al. (17) showed the yield for granulomas to be higher with cell block preparations as performed in our institution, compared with cytological material directly smeared onto slides, on cytospins and on ThinPrep preparations.
Additionally, in a minority of cases, the status of the lymphadenopathy was further confirmed through mediastinoscopy whilst in most of the patients, the reactive nature of the lymphadenopathy was confirmed if the radiological follow-up showed regression of the lymphadenopathy at 6 months. As lymphadenopathy due to sarcoidosis may regress spontaneously, this may have led to an underestimation of the false negative rate which would thus influenced both the sensitivity and NPV. Additionally, we did not include the non-diagnostic EBUS-TBNA, where samples were felt to be inadequate on a per-patient basis.
We had one false positive case whereby epithelioid granulomas were found in three mediastinal nodal stations sampled via EBUS-TBNA in the patient. On further surgical evaluation of the lymph nodes, the histology was in keeping with Hodgkin’s Lymphoma with concurrent granulomas. It is well recognized that non-necrotising sarcoid-like granulomatous inflammation can sometimes found in lymph nodes draining malignancies and in the margins of malignant tumours including lymphoma, predominantly Hodgkin lymphoma, and lung cancer (18). This highlights the importance of maintaining a high index of suspicion and interpreting the cytological findings in clinical context, as well as the need to ensure adequate follow up of such patients when reporting true diagnostic performance of EBUS-TBNA.
Lymphoma
The role of EBUS-TBNA in the diagnosis of lymphoma remains an area of debate. The diagnostic accuracies have been variable in studies to date largely due to heterogeneity in the patient demographics and heterogeneity of lymphoma itself, with higher yield in Non-Hodgkin lymphoma than Hodgkin lymphoma and in relapsed disease (19-26). For instance, a number of studies have examined the test in patients with high suspicion of lymphoma, raising the pre-test probability and hence prevalence of disease (22), whilst others have labelled the test as being true positive even when further invasive sampling was required for further subtyping and grading (20). In our study, there was a very low prevalence of de novo or relapsed lymphoma in the unselected group of patients, such that the sensitivity was low, although the NPV was noted to be high. Our data seems to suggest that in an unselected cohort of patients presenting with IMHL in clinical practice, EBUS can often reveal an alternative diagnosis and hence be a good initial test.
Tissue diagnosis in suspected thoracic and extrathoracic cancer
We also performed another subgroup analysis of patients to assess the ability of EBUS-TBNA to make a tissue diagnosis of cancer. These included patients subjected to EBUS only for diagnostic purposes as well as for both diagnosis and staging in one sitting. Most existing studies (27-51) in lung cancer pertain to the performance of EBUS in correctly staging suspected NSCLC and it is therefore difficult to extrapolate the diagnostic yield of EBUS-TBNA in overall cancer diagnosis in an unselected cohort of patients. Data from meta-analyses show that the overall median sensitivity is 89%, with values ranging from 46% to 97% and that the median NPV is 91%, ranging from 60% to 97% (4). One prospective study examined the utility of EBUS-TBNA in qualitative diagnosis in an unselected cohort of 101 patients with suspected cancer presenting with mediastinal and/or hilar lymphadenopathy, with or without a lung mass (35). The disease prevalence was lower than in our current study group at 60.4%. The authors reported an overall accuracy of 97% and a sensitivity of 95% for making a tissue diagnosis of cancer.
The current study, which also included EBUS-TBNA of suspicious masses (10%), showed a high diagnostic performance in the ability of EBUS-TBNA to get a tissue diagnosis in thoracic and extrathoracic cancer, with an overall diagnostic accuracy of 90%, sensitivity of 88% (95% CI: 85.1–90.5%) and a NPV of 62.0%.
In the current study cohort, only 19% of patients were subjected to further invasive pathological sampling. 50% of the false negatives were based on radiological progression, suggestive of cancer. The precise final histology was not available, which may have influenced the lower diagnostic yield.
Up to 30% of extrapulmonary tumours can be associated with mediastinal or hilar lymph node involvement. In a recent meta-analysis of six studies in a total of 533 patients, the value of EBUS-TBNA in this indication was clear with a pooled sensitivity of 85% and a NPV of 75% (51). The diagnostic performance in extrathoracic cancer in our study group was higher with a sensitivity of 95.7% (95% CI: 78.1–99.9%) and a NPV of 80% (95% CI: 28.4–99.5%). The higher sensitivity may relate to the disease prevalence of 85%, which was higher than in the reported studies (range, 37–68%). The most frequently encountered primary tumours in the meta-analysis were as in our study: head and neck, colorectal, breast, renal, oesophageal, gastric, prostatic, and melanoma.
Staging of suspected or confirmed NSCLC
In the current cohort, patients analysed for staging included those with confirmed or suspected NSCLC. A proportion did not have pathological confirmation of NSCLC and were treated clinically as such for e.g., if N0 on EBUS, they underwent radical radiotherapy with subsequent radiological follow up. The sensitivity for N2 staging was 83.7% (95% CI: 76.2–89.6%) with a NPV of 81.58% (95% CI: 73.2–88.2%) and a diagnostic accuracy of 87.8%. The prevalence of N2 disease was 58%.
The British Thoracic Society Quality Standard was published in 2014 (6), which provides a minimum standard of 88% sensitivity for the staging of EBUS-TBNA in suspected lung cancer. This figure is based on the results of a number of meta-analyses (4) on the role of EBUS-TBNA in staging of suspected cancer. These show an overall median sensitivity of 89%, with values ranging from 46% to 97%, and a median NPV of 91%. However, both sensitivity and NPV are dependent on the disease prevalence, with a lower disease prevalence lowering sensitivity and increasing NPV and vice versa. It is thus essential to present the data on disease prevalence in conjunction with performance measures.
The reasons for lower sensitivity for accurate N2 staging for lung cancer in our study group were examined.
In the current cohort, only 60% underwent mediastinoscopy and thoracotomy and systematic sampling if mediastinoscopy excluded N2 disease. This is in contrast to most published studies on staging, where most patients were subjected to surgical staging if EBUS-TBNA were negative (27-51). The patents included in those studies could be thus considered operable, unlike in our study population, whereby 39% underwent clinical follow up as they were deemed inoperable. This probably reflects real life practice.
If patients without surgical confirmation of negative EBUS-TBNA were excluded, the sensitivity was 91% (95% CI: 88.4–95.4%) and a NPV of 84.3% (95% CI: 73.6–91.9%), with a disease prevalence of 67.4%.
Most of the published studies evaluating EBUS have used a systematic approach to staging (28,29,32-35,37-51). This involves sampling of at least one node per station if deemed detectable and accessible and sampling of several nodes in a station if they are suspicious. Our current practice has been predominantly selective to date, with sampling limited to predominantly radiologically abnormal nodes. This may have resulted in the reduced performance of EBUS TBNA in our study cohort. There is evidence to suggest that systematic mediastinal staging may improve accuracy over a more limited or selective approach, although such data is not available for needle based techniques (52,53).
Our study population also excluded patients with ACCP group A, given the lack of benefit in pathological staging over radiological staging in this subgroup. Published studies have not all excluded patients with ACCP Group A staging, reflecting different disease prevalence in the study populations. Additionally, a number of studies have utilized ROSE (32,33,35,38,39-41,42,44,48,50), which was not available at our centre, other studies which have contributed to the meta-analysis combined both EBUS and endoscopic ultrasonography (EUS).
Although the performance targets set out by the British Thoracic Society (6) and suggested by the recent paper by Evison et al. (7), are helpful in guiding the centers for achieving minimum standards, it is also essential to exercise caution before interpreting local data, as they reflect different study groups with inherently larger heterogeneity than in published studies. In evaluating local performance, extrapolating published evidence to real life data may not be straightforward.
Limitations and strengths of the study
The main limitation of this study was its retrospective nature. The indications of the procedure were not always made clear during the data entry. Thus the CT scans and PET-CT reports of patients had to be retrospectively evaluated and judgment made regarding the indications. This may thus have introduced significant observer bias.
A significant proportion of patients with EBUS-TBNA negative results underwent clinical follow up only, which may have influenced the true performance characteristics of the test in our cohort. For example, if a patient with suspected lung cancer and N2 stage negative on EBUS showed progression of the mediastinal nodes on CT on follow up, this was classed as false negative. The presence of enlarged and reactive hyperplasia associated with cancer would have been an alternative explanation, in which case the results would have been true negative. Conversely, in patients with IMHL, the reactive nature of the lymphadenopathy was confirmed as such if the radiological follow-up showed regression of the lymphadenopathy within 6 months. As lymphadenopathy due to sarcoidosis may regress spontaneously, this may have led to an underestimation of the false negative rate which would thus influence both the sensitivity and NPV.
Nevertheless, as all patients referred consecutively for EBUS-TBNA were included in the study, the study population is therefore representative of unselected patients, reflecting real life practice. The population of patients in this study may be a more accurate reflection of the diverse population that presents to the general respiratory physician in the UK.
Recommendations
The study highlighted some of the difficulties in the retrospective evaluation of large volume data for assessing local performance measures of EBUS-TBNA. Patients are referred for EBUS for various indications. There is a usually a high degree of clinico-radiological heterogeneity amongst patients. There are procedure related factors that can additionally influence the performance of the procedure.
Data capture and analysis
It is essential that for obtaining as accurate information as possible, data is collected prospectively, capturing all relevant clinico-radiological and procedure related factors. For example, the indications need to be clear at the time of the procedure, including the following main groups: investigation of IMHL, staging only of suspected NSCLC, diagnosis only of suspected cancer, diagnosis and staging of suspected cancer in one sitting, genetic testing only. Amongst patients subjected to staging purposes, patients should be grouped further into relevant ACCP categories for further subgroup analysis. For the analysis of data, we recommend the final results are based on at least 6 months clinical, microbiological, pathological and radiological follow up in all patients. The denominator for staging should be the overall number of patients with N2/3 nodal metastases (even in those lymph node stations inaccessible with EBUS) and consideration should be made to also include the non-diagnostic samples. Similarly, for diagnostic purposes, the denominator should include the ability of the procedure to diagnose cancer, including in those with masses and nodes not accessible to the EBUS-TBNA. Patients, in whom EBUS-TBNA raises the possibility of lymphoma but require further pathological sampling for subtyping and grading, should be identified as false negative.
Technical tips
In our study cohort, patients underwent selective staging of abnormal nodes as opposed to systematic staging of all accessible nodes. Extrapolation from surgical data would suggest that the most thorough level of needle-based staging should involve sampling of at least one node per station if deemed detectable and accessible and sampling of several nodes in a station if they are suspicious (54). In addition, it is essential that a minimum of three N3/N2 stations are sampled per patient for optimal accuracy (7). A minimum of three passes per station is also recommended although there is data to suggest adequacy of samples if 1 out of 2 samples contains cores (46). However, in the era of molecular profiling, the need for a higher volume sample should be considered to facilitate tests such as EGFR and ALK testing (55).
Additionally, in most centers, there is likely to be more than 1 operator contributing to the EBUS service. In order to examine the influence of any operator-related factors on local data, further evaluation of individual performances may also be helpful in identifying variation in practice and areas for improvement.
Overall, we believe that this retrospective cohort study of a large volume of patients represents real life practice and provides an accurate representation of the typical cohort of patients referred in for EBUS-TBNA to the general respiratory physician in UK. Our study highlights the pitfalls in collecting and analyzing data but also demonstrates how they can be used to improve the service performance. As we enter an era where EBUS centers are likely to undergo central accreditation to ensure that they meet minimum standards, we recommend that local measures are in place to facilitate robust data collection and a continuous review of operating practices and performance.
Acknowledgements
The authors thank Dr. Ben Prudon (Consultant Respiratory Physician, Department of Respiratory Medicine, North Tees and Hartlepool NHS Foundation Trust, Stockton on Tees, United Kingdom) and Dr. Graham Miller (Consultant Respiratory Physician, Department of Respiratory Medicine, North Tees and Hartlepool NHS Foundation Trust, Stockton on Tees, United Kingdom).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethics approval waived as this was deemed to be a service evaluation by our local R&D department.
References
- Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J 2009;33:1156-64. [Crossref] [PubMed]
- NICE lung cancer guidelines. Available online: https://www.nice.org.uk/guidance/cg121/chapter/1-Guidance.
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer diagnosis and management of lung cancer, 3rd ed: American college of chest physicians Evidence-Based clinical practice guidelines. Chest 2013;143:E211-50.
- Cusworth K, O'Dowd E, Hubbard R, et al. Variation in lung cancer resources and workload: results from the first national lung cancer organisational audit. Thorax 2015;70:1001-3. [Crossref] [PubMed]
- British Thoracic Society. Quality Standards for Flexible Bronchoscopy in Adults. British Thoracic Society Reports 2014;6(5).
- Evison M, Crosbie P, Navani N, et al. How should performance in EBUS mediastinal staging in lung cancer be measured? Br J Cancer 2016;115:e9. [Crossref] [PubMed]
- Natu S, Hoffman J, Siddiqui M, et al. The role of endobronchial ultrasound guided transbronchial needle aspiration cytology in the investigation of mediastinal lymphadenopathy and masses,the North Tees experience. J Clin Pathol 2010;63:445-51. [Crossref] [PubMed]
- Evison M, Crosbie PA, Morris J, et al. A study of patients with isolated mediastinal and hilar lymphadenopathy undergoing EBUS-TBNA. BMJ Open Resp Res 2014;1:e000040. [Crossref] [PubMed]
- Navani N, Lawrence DR, Kolvekar S, et al. Endobronchial ultrasound-guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial. Am J Respir Crit Care Med 2012;186:255-60. [Crossref] [PubMed]
- Agarwal R, Srinivasan A, Aggarwal AN, et al. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: a systematic review and meta-analysis. Respir Med 2012;106:883-92. [Crossref] [PubMed]
- Trisolini R, Lazzari Agli L, Tinelli C, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for diagnosis of sarcoidosis in clinically unselected study populations. Respirology 2015;20:226-34. [Crossref] [PubMed]
- Mcmanus TE, Haydock DA, Alison PM, et al. Isolated mediastinal adenopathy: the case for mediastinoscopy. Ulster Med J 2008;77:97-101. [PubMed]
- Porte H, Roumilhac D, Eraldi L, et al. The role of mediastinoscopy in the diagnosis of mediastinal lymphadenopathy. Eur J Cardiothorac Surg 1998;13:196-9. [Crossref] [PubMed]
- Trisolini R, Cancellieri A, Tinelli C, et al. Transbronchial needle aspiration in sarcoidosis: yield and predictors of a positive aspirate. J. Thorac Cardiovasc Surg 2008;135:837-42. [Crossref] [PubMed]
- Bilaçeroğlu S, Perim K, Günel O, et al. Combining transbronchial aspiration with endobronchial and transbronchial biopsy in sarcoidosis. Monaldi Arch Chest Dis 1999;54:217-23. [PubMed]
- Schwartz LE, Griffin AC, Baloch Z. Cell block interpretation is useful in the diagnosis of granulomas on cytology. Diagn Cytopathol 2012;40:939-40. [Crossref] [PubMed]
- Hollingsworth HC, Longo DL, Jaffe ES. Small noncleaved cell lymphoma associated with florid epithelioid granulomatous response. A clinicopathologic study of seven patients. Am J Surg Pathol 1993;17:51-9. [Crossref] [PubMed]
- Iqbal S, DePew ZS, Kurtin PJ, et al. Endobronchial ultrasound and lymphoproliferative disorders: a retrospective study. Ann Thorac Surg 2012;94:1830-4. [Crossref] [PubMed]
- Steinfort DP, Conron M, Tsui A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of suspected lymphoma. J Thorac Oncol 2010;5:804-9. [Crossref] [PubMed]
- Moonim MT, Breen R, Fields PA, et al. Diagnosis and subtyping of de novo and relapsed mediastinal lymphomas by endobronchial ultrasound needle aspiration. Am J Respir Crit Care Med 2013;188:1216-23. [Crossref] [PubMed]
- Kennedy MP, Jimenez CA, Bruzzi JF, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax 2008;63:360-5. [Crossref] [PubMed]
- Marshall CB, Jacob B, Patel S, et al. The utility of endobronchial Ultrasound-Guided transbronchial needle aspiration biopsy in the diagnosis of mediastinal lymphoproliferative disorders. Cancer Cytopathol 2011;119:118-26. [Crossref] [PubMed]
- Ko HM, Santos GD, Darling G, et al. Diagnosis and subclassification of lymphomas and Non-Neoplastic lesions involving mediastinal lymph nodes using endobronchial Ultrasound-Guided transbronchial needle aspiration. Diagn Cytopathol 2013;41:1023-30. [Crossref] [PubMed]
- Talebian Yazdi M, von Bartheld B, Waaijenborg FG, et al. Endosonography for the diagnosis of malignant lymphoma presenting with mediastinal lymphadenopathy. J Bronchology Interv Pulmonol 2014;21:298-305. [Crossref] [PubMed]
- Grosu HB, Iliesiu M, Caraway NP, et al. Endobronchial Ultrasound Guided Transbronchial Needle Aspiration Accurately Diagnoses and Subtypes Lymphoma. Ann Am Thorac Soc 2015;12:1336-44. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Krasnik M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest 2008;133:887-91. [Crossref] [PubMed]
- Wallace MB, Pascual JM, Raimondo MA, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA 2008;299:540-6. [Crossref] [PubMed]
- Herth FJ, Krasnik M, Kahn N, et al. Combined Endoscopic-Endobronchial Ultrasound-Guided Fine-Needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest 2010;138:790-4. [Crossref] [PubMed]
- Szlubowski A, Zieliński M, Soja J, et al. A combined approach of endobronchial and endoscopic ultrasound-guided needle aspiration in the radiologically normal mediastinum in non-small-cell lung cancer staging - a prospective trial. Eur J Cardiothorac Surg 2010;37:1175-9. [Crossref] [PubMed]
- Lee BE, Kletsman E, Rutledge JR, et al. Utility of endobronchial ultrasound–guided mediastinal lymph node biopsy in patients with non–small cell lung cancer. J Thorac Cardiovasc Surg 2012;143:585-90. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective con-trolled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]
- Memoli JS, El-Bayoumi E, Pastis NJ, et al. Using endobronchial ultrasound features to predict lymph node metastasis in patients with lung cancer. Chest 2011;140:1550-6. [Crossref] [PubMed]
- Cetinkaya E, Seyhan EC, Ozgul A, et al. Efficacy of con-vex probe endobronchial ultrasound(CP-EBUS)assisted transbronchial needle aspiration for mediastinal staging in non-small cell lung cancer cases with mediastinal lymphadenopathy. Ann Thorac Cardiovasc Surg 2011;17:236-42. [Crossref] [PubMed]
- Ye T, Hu H, Luo X, et al. The role of endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) for qualitative diagnosis of mediastinal and hilar lymphadenopathy: a prospective analysis. BMC Cancer 2011;11:100. [Crossref] [PubMed]
- Steinfort DP, Hew MJ, Irving LB. Bronchoscopic evaluation of the mediastinum using endobronchial ultrasound: a description of the first 216 cases carried out at an Australian tertiary hospital. Intern Med J 2011;41:815-24. [Crossref] [PubMed]
- Hwangbo B, Lee GK, Lee HS, et al. Transbronchial and transesophageal Fine-Needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest 2010;138:795-802. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Eloubeidi MA, et al. The true false negative rates of esophageal and endobronchial ultrasound in the staging of mediastinal lymph nodes in patients with Non-Small cell lung cancer. Ann Thorac Surg 2010;90:427-34. [Crossref] [PubMed]
- Sun W, Zervos M, Pass H, et al. The diagnostic value of endobronchial Ultrasound-Guided needle biopsy in lung cancer and mediastinal adenopathy. Cancer Cytopathol 2008;114:415-6.
- Nakajima T, Yasufuku K, Nakajima M, et al. Endobronchial Ultrasound-Guided transbronchial needle aspiration for lymph node staging in patients with non-small cell lung cancer in nonoperable patients pursuing radiotherapy as a primary treatment. J Thorac Oncol 2010;5:606-11. [Crossref] [PubMed]
- Gilbert S, Wilson DO, Christie NA, et al. Endobronchial ultrasound as a diagnostic Tool in patients with mediastinal lymphadenopathy. Ann Thorac Surg 2009;88:896-900; discussion 901-2. [Crossref] [PubMed]
- Fielding D, Windsor M. Endobronchial ultrasound convex-probe transbronchial needle aspiration as the first diagnostic test in patients with pulmonary masses and associated hilar or mediastinal nodes. Intern Med J 2009;39:435-40. [Crossref] [PubMed]
- Hwangbo B, Kim SK, Lee HS, et al. Application of endobronchial ultrasound-guided transbronchial needle aspiration following integrated PET/CT in mediastinal staging of potentially operable non-small cell lung cancer. Chest 2009;135:1280-7. [Crossref] [PubMed]
- Rintoul RC, Tournoy KG, El Daly H, et al. EBUS-TBNA for the clarification of PET positive intra-thoracic lymph nodes-an international multi-centre experience. J Thorac Oncol 2009;4:44-8. [Crossref] [PubMed]
- Bauwens O, Dusart M, Pierard P, et al. Endobronchial ultrasound and value of PET for prediction of pathological results of mediastinal hot spots in lung cancer patients. Lung Cancer 2008;61:356-61. [Crossref] [PubMed]
- Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest 2008;134:368-74. [Crossref] [PubMed]
- Herth FJ, Rabe KF, Gasparini S, et al. Transbronchial and transoesophageal (ultrasound-guided) needle aspirations for the analysis of mediastinal lesions. Eur Respir J 2006;28:1264-75. [Crossref] [PubMed]
- Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006;130:710-8. [Crossref] [PubMed]
- Yasufuku K, Chiyo M, Koh E, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer 2005;50:347-54. [Crossref] [PubMed]
- Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122-8. [Crossref] [PubMed]
- Yang B, Li F, Shi W, et al. Endobronchial ultrasound-guided transbronchial needle biopsy for the diagnosis of intrathoracic lymph node metastases from extrathoracic malignancies: a meta-analysis and systematic review. Respirology 2014;19:834-41. [Crossref] [PubMed]
- Detterbeck FC. Integration of mediastinal staging techniques for lung cancer. Semin Thorac Cardiovasc Surg 2007;19:217-24. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non–small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Detterbeck F, Puchalski J, Rubinowitz A, et al. Classification of the thoroughness of mediastinal staging of lung cancer. Chest 2010;137:436-42. [Crossref] [PubMed]
- Yarmus L, Akulian J, Gilbert C, et al. Optimizing endobronchial ultrasound for molecular analysis. How many passes are needed? Ann Am Thorac Soc 2013;10:636-43. [Crossref] [PubMed]


