Survival and prognostic factors following pulmonary metastasectomy for sarcoma
Introduction
After resection of primary tumors, metastasectomy is the most frequent surgical operation involving the lung. Based on the report of the International Registry of Lung Metastases, the most frequent tumors metastasizing to the lung are epithelial tumors (44% of all lung metastasis) followed by sarcomas (42%) (1).
Sarcomas represent a rare (1% of all cancers) and heterogeneous group of tumors, arising both from soft tissues and bones (2). Multimodality treatments allow to reach 5-year survival rates from 30% to 80% for osteosarcomas and 15% to 90% for soft-tissue sarcomas (3).
These tumors show a high propensity to metastasize to the lung; about 20% of patients diagnosed with soft-tissue sarcoma and 40% of those with a primary bone sarcoma will develop pulmonary metastases at some point in the course of their disease, with the lung being the only site of disease in 19% of cases (4). Any subtype of sarcoma is potentially able to metastasize to the lung, although the most frequent histology is osteosarcoma, followed by synovial sarcoma and liposarcoma (5).
The development of pulmonary metastases has negative impact on prognosis, with most of the untreated patients dying within 6–11 months from diagnosis. Chemotherapy remains the standard treatment in metastatic sarcomas, but when it is performed alone, it shows poor improvement in survival rates. On the other hand, surgical therapy seems to be effective in prolonging survival among patients with resectable disease, with 5-year survival rates ranging from 15% to 50.9% (6-26).
Introduction of minimally invasive approaches and parenchymal sparing techniques have led to decreased morbidity and possibility to perform repeated operations in cases of relapsing disease. This is particularly important in metastatic sarcomas, where about 64% of patients undergoing pulmonary metastasectomy will experience a new relapse of disease (1).
Moreover, new adjunctive therapies have gained attention, such as radiofrequency ablation (RFA) or stereotactic body radiation therapy, and offered new therapeutic options in patients unfit for surgery (4,27,28).
Treatment of metastatic disease continues to evolve also with a better knowledge of the clinical and pathological features of metastatic tumors. Particularly, the theory of oligometastases and oligorecurrence has gained attention. It corresponds to a limited number of typically non-bulky metastases, which seem to have different natural histories and better prognosis than multiple metastatic diseases. Therefore, it may be considered an intermediate stage between local and metastatic disease that should be managed more aggressively than standard palliative care (27).
To date, there is no prospective randomized study proving the effectiveness of pulmonary metastatic surgery, not only for sarcomas (29). Current knowledge is based only on retrospective case-series, with related selection biases. As pointed out by some authors, improved survival may be related to a less malignant tumor biology or to the fact that patients undergoing surgical resection have intrinsically favorable characteristics than those treated with chemotherapy alone (30). However, a randomized controlled trial seems unfeasible, as it would be considered unethical to exclude patients from local ablation treatments (31). Retrospective case-series have identified several clinicopathological parameters as prognostic factors for long-term survival, such as histological grade, tumor size, number of nodules, disease-free interval (DFI) and resectability (16,31).
Despite the formal lack of evidence, surgery represents a well-established treatment option in metastatic sarcoma, and a joint study from MD Anderson and Memorial Sloan-Kettering Cancer Centers demonstrated that it is more cost-effective compared to chemotherapy (32).
Selection criteria for surgery
Preconditions to met, in order to undergo a potentially curative pulmonary metastasectomy, are essentially those proposed by Thomford in 1965 (33): feasibility of a complete resection of all known disease; tolerable general and functional risk; primary tumor under control; no evidence of extrathoracic disease.
The presence of extrathoracic spread is the main reason for exclusion from surgery. Advances in the sensitivity of imaging techniques, especially the development of positron emission tomography (PET), have increased the proportion of patients discovered with inoperable disease (34), leading to a better selection of surgical candidates. Functional risk assessment should follow general guidelines for determining operability in lung resection (35).
Ultimately, the decision to proceed with surgery has to take into account the relative risks and benefits of all other therapeutic options. Thus, it is advisable that each case be discussed in a multidisciplinary setting, with a dedicated oncologist, surgeon, and radiotherapist.
Operative technique
Classically, open surgical approaches have been used for resection of pulmonary metastases. Standard thoracotomy is the most commonly used access for unilateral disease or as staged thoracotomies for bilateral lesions. Sternotomy and clamshell incisions have been described for bilateral disease. Advantages of open approaches are an adequate access and visualization of the lung surface and the possibility to perform bimanual palpation of the parenchyma to identify deep, small nodules, sometimes not detected by imaging techniques.
Video-assisted thoracoscopic surgery (VATS) has become an increasingly popular method for resection of pulmonary lesions; however, its use in the management of lung metastases is controversial.
Advantages of a minimally invasive approach include limited skin incision, decreased postoperative pain and shorter hospital stay. The main criticism which has been raised, on the other hand, is that the inability to perform bimanual palpation of the lung may result in failure to detect all lesions, as demonstrated in earlier studies (36). However, it is still unclear whether removal of the additional lesions detectable by bimanual palpation results in a survival benefit (37); moreover, improvement in thoracic imaging and novel techniques for preoperative localization of the lesions, such as CT-guided percutaneous implantation of a hook wire, may increase the number of lesions detectable through a VATS approach (38). Lastly, given the tendency of sarcoma metastases to recur, VATS may better preserve the patient ability to undergo possible repeated resections.
Gossot et al. demonstrated that, in patients with a maximum of two small lesions amenable to wedge resection, there is no statistically significant survival difference among patients undergoing VATS versus open thoracotomy. The authors concluded that, in selected patients, VATS is equally effective and less invasive than open surgery (39). This opinion is supported by other studies (40,41).
Concerning the extent of resection, in surgery for metastatic disease, parenchyma-sparing approaches to preserve pulmonary function are critically important. Since the majority of sarcoma metastases are peripherally located, wedge resection and segmentectomy are the most commonly performed procedure. Lobectomy, and in selected cases even pneumonectomy, is indicated for centrally located or multiple lesions (42). A circumferential margin of at least 0.5 to 1.0 cm of normal lung tissue is recommended (43), in cases of larger lesions or pleural penetration (which means for the surgeon, superficial pleura-attached metastases), larger margins have been suggested (26). Some investigators have employed a specifically designed laser technology for resection of lung metastases (44). According to the authors, this technique offers the possibility of resecting a large number of metastases, even in cases of bilateral disease or centrally located lesions, facilitating the achievement of a complete resection and maximizing the sparing of lung parenchyma.
Sarcomas are believed to spread primarily through a hematogenous route, thus, unlike in other secondary tumors of the lung, lymph node involvement is rarely seen, except for some histological subtypes as rhabdomyosarcomas, synovial sarcomas and epithelioid sarcomas (31). The only study in which a systematic lymphadenectomy was performed concurrently with the resection of lung metastases identified hilar or mediastinal lymph node involvement in 24% of cases, without a significant impact on survival (18). Therefore, lymphadenectomy is not routinely recommended during resection of pulmonary metastases from sarcoma (2).
Timing of surgery in pulmonary metastatic resection is still matter of debate. Some authors prefer an aggressive approach with resection as soon as metastases are identified. Others allow for a time interval between identification and surgical resection. Clearly, leaving a time interval may not only allow detection of new nodules and prevent overlooking very small metastases, but also may provide clues on the nodules doubling time and on the response to a chemotherapy treatment (29).
Chemotherapy
There is no consensus standard of care concerning chemotherapy in metastatic sarcoma; earlier clinical trials including sarcomas were frequently confounded by lumping together results from biologically disparate subtypes, which are characterized by different degrees of chemosensitivity (45). In the past years, a more thoughtful, histology-tailored approach has led to improved outcomes in patients with more aggressive histologies, such as Ewing sarcoma, osteosarcoma and non-pleomorphic rhabdomyosarcoma, whereas gains have been more modest in other soft-tissue sarcoma subtypes (46).
Since most patients will not be cured with aggressive multimodality therapy, quality of life must be carefully considered when recommending a treatment program. Currently, multi-agent anthracycline-based combination therapy is reserved for patients with a good performance status, relatively chemosensitive histology, and patients who are either symptomatic or are being considered for surgical resection (47).
Recently published trials have demonstrated promising results with newer cytotoxic agents, including eribulin, trabectedin, and aldoxorubicin. On the contrary, therapeutic options involving targeted therapy or immune therapy are still in their infancy (46).
The effectiveness of conventional chemotherapy as an adjunct to surgical metastasectomy has not been demonstrated, yet. There’s an important selection bias in surgical case-series, in that chemotherapy is usually reserved to those cases with more aggressive disease. Furthermore, different treatment protocols and mixed histologies represent two important confounding variables. A study on soft-tissue sarcoma patients from the Memorial Sloan-Kettering Cancer Center, which specifically compared the two cohorts of patients receiving pulmonary metastasectomy alone or in combination with perioperative chemotherapy, did not demonstrate a better outcome in the latter group, even after adjustment on propensity scores accounting for selection bias (48).
In osteosarcoma patients, another study from the Istituto Ortopedico Rizzoli have reported favorable outcomes (5-year event-free survival =27.4%) in the 91 patients with synchronous lung metastases treated with neoadjuvant chemotherapy and simultaneous complete resection of primary and metastatic lesions; however, these results were not comparable with an equally-matched control group (49).
Despite the unproven benefit on survival, chemotherapy, administered in a neoadjuvant setting, might offer the benefit to better assess tumor biology and response to treatment prior to surgical resection. The role of the response to chemotherapy as a prognostic factor will be discussed below.
Radiation therapies
Many sarcoma subtypes have been traditionally considered radio-resistant, such as liposarcomas and osteosarcomas. However, there are new evidences of promising results in the treatment of metastatic sarcomas with radiation therapies.
Whole lung irradiation is performed only in case of Ewing sarcoma, which is uniquely radiosensitive, with improvement in survival and little toxicity (50).
Stereotactic Body Radiation Therapy is a high dose, hypofractionated, focused radiation therapy. For its characteristics, it potentially seems to overcome the radio-resistance of sarcomas. Moreover, it can be performed in patients unfit for surgery or in cases of indolent or progressive disease or bilateral disease. Several studies have shown good survival rates, however, long-term results are lacking (4,51).
CT-guided RFA uses high frequency alternating current that causes high temperatures around the inserted electrode, with intratumoral necrosis (51). There are just three studies describing the experience with RFA for lung metastasis, showing 3-year survival rates between 29% and 85% (52-54). A frequent complication of this procedure is pneumothorax (68% of cases) with the need of chest tube positioning in more than half of the patients (53). Although it may be considered an alternative for patients unfit for surgery, RFA shows some limitations, especially for centrally located nodules or nodules in close proximity to large vessels (2).
Novel therapies
Yellin and colleagues described a series of patients undergoing surgical resection and hyperthermic pleural perfusion with cisplatin for malignancies with pulmonary and pleural involvement. Among these patients, two had sarcoma metastases and one had Ewing sarcoma. While the latter died 6 months after operation, the other two patients survived for 15 and 54 months (55).
Recently, Den Hengst and colleagues performed a phase II clinical trial with pulmonary metastasectomy associated to isolated lung perfusion with melphalan. Compared with literature data, it showed an improvement in local control of disease and comparable results in terms of overall survival, disease-free survival and time to progression (56).
Prognostic factors after pulmonary metastasectomy
A summary of recent surgical series of pulmonary metastasectomy for sarcoma is provided in Table 1. Comparison between studies is difficult owing to significant heterogeneity in the histologic subset analysed, selection of patients, and measurement of outcome parameters.
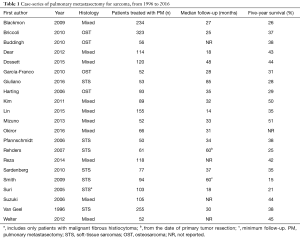
Full table
They report 5-year overall survival rates after resection that range from 15% to 50.9% and evaluate the role of different prognostic factors on survival.
Some of these, namely DFI, complete resection, and number of nodules, are well-known prognosticators of survival, common to every type of secondary tumour of the lung, as identified by the International Registry of Lung Metastases (1). The role of other histologic, clinical and treatment-related features, occasionally producing a significant survival difference in surgical retrospective case-series, is less clearly defined.
A thorough knowledge of survival outcomes and prognostic factors helps to better guide the selection of patients for surgery and to evaluate the most appropriate pattern of care in a multidisciplinary setting.
Age and gender
Patients affected by sarcoma metastases are usually young and without particular comorbidities, which is a reason why many authors recommend an aggressive surgical approach (2). This is especially true for osteosarcoma patients, whose outcomes after metastasectomy are often drawn from series focusing on the paediatric population (8,13). In a recent systematic review, Treasure and colleagues found that the average age of patients with bone sarcoma undergoing metastasectomy was 17 years, while it was 46 for soft-tissue sarcoma patients. According to the same study, there is also a significant difference in gender distribution, with a male predominance (65%) for osteosarcoma patients, while they represent 50% of the soft-tissue sarcoma population. The authors affirm that these differences in age and sex preclude meaningful amalgamation of outcome data following pulmonary metastasectomy for bone and soft-tissue sarcoma (30).
Increasing age resulted an adverse prognostic factor in several studies, including the report from the multi-institutional database of the European Organization for Research and Treatment of Cancer—Soft Tissue and Bone Sarcoma Group (25), on patients with soft-tissue sarcoma, and other case-series with mixed histologies (10,15,26).
In general, advanced age is considered only a relative contraindication to pulmonary metastasectomy, and since chemotherapy is also poorly tolerated in this age group, the relative risks and benefits for these patients must be carefully considered (10).
Sex, on the other hand, is not a prognostic factor for survival, with the exception of one study, on osteosarcoma patients, which found that male subjects have a worse prognosis compared to females (8).
Histology
The first broad distinction must be made between bone sarcomas and soft-tissue sarcomas.
As already mentioned, there is some discrepancy in literature being almost half of the most recent retrospective studies on pulmonary metastasectomy for sarcoma constituted by series with mixed histologies, while the other half represented by series containing either osteosarcoma or soft-tissue sarcoma patients (Table 1). Survival is thought to be slightly better for patients with osteosarcoma, with 5-year overall survival rate of 34% compared to 25% for soft-tissue sarcoma according to a recent systematic review (30). In the studies with mixed histologies however, the difference in survival between osteosarcoma and soft-tissue sarcoma fails to reach significance, except occasionally (15), probably due to the small sample size and the presence of confounding covariates.
Soft-tissue sarcomas, in turn, comprise more than 50 histologic variants (57). The most frequent subtypes metastasizing to the lungs are leiomyosarcoma (LMS), malignant fibrous histiocytoma (MFH, also known as undifferentiated pleomorphic sarcoma), synovial sarcoma and liposarcoma (5,25). Again, none of these subtypes constitutes itself an independent prognostic factor for survival, although, in some studies, patients with LMS metastases had a favourable outcome compared to other histologies (5,6,15). Burt and colleagues corroborated this finding: in their series, the LMS cohort of patients exhibited a significant overall survival advantage after pulmonary metastasectomy (median survival 69.9 months versus 23.9 months for other sarcoma subtypes), presented with fewer pulmonary lesions and had a trend toward fewer involved lobes than patients with non-LMS pulmonary metastases. The authors conclude that LMS may represent a histologic subtype with less aggressive tumour biology (58).
Concerning the survival outcomes of patients with other histologic subtypes, a survival benefit has been reported occasionally in patients with rhabdomyosarcoma (59) and alveolar soft part sarcoma (60), while conflicting results have been reported on MFH patients (24,61). In the study by Billingsley and colleagues, patients with pulmonary metastases of malignant peripheral nerve sheath tumours and liposarcoma histology had a reduced survival (5).
Grade and other histologic features
Most pulmonary metastases arise from high-grade primary tumours. Billingsley and colleagues demonstrated that the incidence of pulmonary metastases within each histologic subtype of soft-tissue sarcoma correlates with the incidence of high-grade lesions within that group (5). The same study found low-grade primary tumour as an independent favourable prognostic factor for survival in patients with pulmonary metastases from soft-tissue sarcoma, surmising that low-grade sarcomas, although demonstrating a similar metastatic potential to high-grade lesions, appear to have a more indolent rate of disease progression. Some studies have confirmed this finding also in the subset of patients undergoing pulmonary metastasectomy for soft-tissue sarcoma metastases (12,25,60). The prognostic value of grade however, is not always evident, and other authors, conversely, propose that once pulmonary metastases have already developed, the importance of grade for post-metastasectomy survival greatly diminishes (22).
Beside tumour grade, other histopathological parameters have been investigated for their prognostic and surgical implications. In particular, Welter and colleagues have described the histologic growth patterns of lung metastases from sarcoma, and identified interstitial spread, vascular infiltration, satellite nodules and lymphangitic spread as aggressive histological growth characteristics (62). In a subsequent study, the same authors observed that the interstitial growth pattern was a risk factor for death after metastasectomy, while pleural penetration and partial tumour regression after chemotherapy were other histo-morphological characteristics associated with increased risk of local intrapulmonary recurrence (26). Other investigators, in a smaller cohort of 24 patients with liposarcoma metastases, noted a correlation between aggressive histological patterns in the lung and a poorer disease outcome (63). These interesting findings warrant further investigation in larger series.
DFI
The DFI, defined as the time interval between complete resection of primary tumour and diagnosis of pulmonary metastases, is a well-known prognosticator of survival after metastasectomy (1). Its value in predicting survival after resection of sarcomatous lung metastases has been confirmed by a number of studies (6,7,9,13-15,21,22,25), despite the different cut-off proposed by the various authors, ranging from 12 to 30 months.
A common consensus concerning the time interval after which lung metastasectomy is indicated does not exist. Median DFI between studies varies widely from 11 to 34 months, which probably reflects different policies of patient selection depending on this parameter (Table 2). Likewise, the presence of synchronous metastases, which by definition have no DFI, is not considered an absolute contraindication for pulmonary metastasectomy by many of the authors, who report percentages as high as 37.5% of patients operated with synchronous metastatic disease (8).
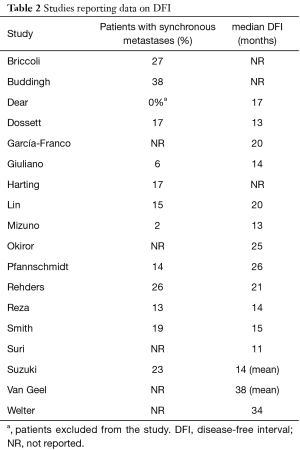
Full table
A short DFI or synchronous disease, on the other hand, prompts consideration to neoadjuvant treatment before pulmonary resection, with the intent to create a window of time to monitor tumour progression, and better evaluate the benefit of surgical treatment (64).
Extrapulmonary disease
In the field of pulmonary metastasectomy for colorectal cancer, there’s a growing body of literature reporting satisfying outcomes after combined resection of hepatic and pulmonary metastases (65). In metastatic sarcoma, on the contrary, the traditional surgical criteria proposed by Thomford (33) (i.e., all the disease outside the chest should be controlled prior to pulmonary resection) is still held true by many authors (42), who specifically include it among exclusion criteria for surgery in their study population (7,11,14,18). Other Institutions, however, with the intent to provide an aggressive surgical treatment to a generally young and fit patient population, perform pulmonary resection also in patients with known extrapulmonary disease (6). While most retrospective series report unfavourable outcomes in these subjects (20,23), Blackmon and colleagues have shown that, if prior or synchronous extrathoracic disease can be completely resected, similar survival rates to those of patients with isolated lung metastases can be achieved (37.8 and 35.5 months of median survival respectively in the two groups) (6). The authors conclude that extrapulmonary metastases should no longer be viewed as an absolute contraindication for surgery. However, this still represents an area of controversy.
Response to chemotherapy
The radiologic response to neoadjuvant chemotherapy has also been investigated for its role as prognostic factor in patients undergoing pulmonary metastasectomy (64,66). Stephens et al. in particular, showed that patients without tumour progression while on chemotherapy had a median survival after metastasectomy of 35.5 months, compared with 17.2 months for those with progression of disease. Similar findings have been obtained by assessing pathologic response, measured as percentage of tumour necrosis in resected specimens of sarcoma metastases (8,13,66). These results suggest that neoadjuvant chemotherapy, in selected patients, can help to assess tumour biology and response to treatment, in order to better evaluate the benefit of subsequent therapeutic options.
Number of metastases, laterality and size
The number of metastatic foci is a measure of the tumour burden, and as such, this parameter is often used in patient selection for surgery, or in some Institutions, for neoadjuvant chemotherapy (19,64). However, there is no agreement about the maximum number of pulmonary nodules indicated to undergo surgical resection, and the median number of resected nodules can vary from one (12) to five (19) in different studies.
Its role as a prognostic factor has been shown in several surgical series (6,8,10,16,21,23). Other measures of tumour burden, such as unilateral versus bilateral disease (14), size of the biggest nodule (9,26) or total size of resected nodules (67), have less frequently been found to be prognosticators of survival as well.
This association however is not present in many studies, and some authors have suggested that whenever a complete resection is achieved, measures of tumour burden have a reduced effect on survival (19).
Completeness of resection
The feasibility of a complete resection is considered a main selection criterion when evaluating a patient for pulmonary metastasectomy, and most of the studies confirm its role as a determinant prognostic factor for long-term survival.
In the report from the IRLM, Pastorino and colleagues demonstrated that patients in which a complete resection was not achieved had a median survival of 14 months, which was worse than that of the subjects who had the other two main unfavourable prognostic factors combined (DFI <36 months and multiple metastases, median survival =24 months) (1).
Similarly, Billingsley et al. found that, among patients with soft-tissue sarcoma lung metastases, those with incompletely resected disease had only a marginal statistical survival difference compared with subjects treated with non-resectional therapy (median survival of 16.4 and 11.2 months, respectively), whereas completely resected patients had a significantly better outcome (33.5 months) (5).
It is important to acknowledge that, although complete resection in metastatic sarcoma is almost invariably required for long-term survival, the disease will recur in the majority of cases (22), and that a definitive cure can occur only thanks to a combination of different factors, such as the host histology, tumour spread, response to systemic therapy and surgical resection (21).
Repeat metastasectomy
Sarcomas have a typical intrathoracic pattern of relapse accounting for 66% of all recurrences after pulmonary metastasectomy, significantly different from that of other tumours that re-recur mainly distant. Accordingly, the proportion of relapsing patients who undergo a second pulmonary metastasectomy is significantly higher in sarcomas then in any other tumour type (53% versus 28% in epithelial tumours) (1).
Several studies report a significantly improved 5-year survival rate in re-resected patients compared to patients with a single resection (6,14,19,68,69). This is most likely due to the highly-selected patient cohort, with favourable tumour biology, who meet selection criteria for surgery after each subsequent relapse.
Weiser and colleagues, in their cohort of 86 re-resected patients, reported a median disease specific survival of 42.8 months from the date of the second metastasectomy. Completeness of resection, again, resulted in the main determinant of survival, with a median disease specific survival of 51 months for completely resected patients, compared with 6 months in patients with an incomplete re-resection (70). Similar studies yielded comparable survival outcomes (28,68,69). Beside completeness of resection, the study by Weiser et al. identified three other preoperative prognostic factors: number of nodules, metastasis size and grade of the primary tumour, while other investigators have found the DFI between the first and second metastatic event to be another independent prognostic factor (6,11).
These results suggest that repeat metastasectomy appears beneficial in patients who can be completely resected, and can lead to long-term survival.
Conclusions
Despite the lack of randomized controlled trials, the results obtained by retrospective case-series indicate that pulmonary metastasectomy for sarcomatous disease is a valid treatment option, especially in patients with favourable prognostic factors.
Given the relative lack of effective systemic therapies, on the basis of available retrospective data, it seems legitimate to consider surgery also in patients who show unfavourable clinical features, such as a large tumour burden, synchronous metastases or resectable extrathoracic disease, as long as a complete resection is deemed possible. Furthermore, the same aggressive approach should be warranted to patients who experience a resectable pulmonary relapse, since there’s evidence that long-term survival is possible even after multiple redo operations.
Multimodality therapies, in particular neoadjuvant chemotherapy and other ablative techniques, can improve the quality of care for patients affected by metastatic sarcoma. Future directions include the development of hybrid treatments and the implementation of new, more effective chemiotherapy agents.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Kon Z, Martin L. Resection for thoracic metastases from sarcoma. Oncology 2011;25:1198-204. [PubMed]
- Stiller CA, Trama A, Serraino D, et al. Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer 2013;49:684-95. [Crossref] [PubMed]
- Digesu CS, Wiesel O, Vaporciyan AA, et al. Management of sarcoma metastases to the lung. Surg Oncol Clin N Am 2016;25:721-33. [Crossref] [PubMed]
- Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg 1999;229:602-10; discussion 610-2. [Crossref]
- Blackmon SH, Shah N, Roth JA, et al. Resection of pulmonary and extrapulmonary sarcomatous metastases is associated with long-term survival. Ann Thorac Surg 2009;88:877-84; discussion 884-5. [Crossref] [PubMed]
- Briccoli A, Rocca M, Salone M, et al. High grade osteosarcoma of the extremities metastatic to the lung: long-term results in 323 patients treated combining surgery and chemotherapy, 1985-2005. Surg Oncol 2010;19:193-9. [Crossref] [PubMed]
- Buddingh EP, Anninga JK, Versteegh MI, et al. Prognostic factors in pulmonary metastasized high-grade osteosarcoma. Pediatr Blood Cancer 2010;54:216-21. [PubMed]
- Dear RF, Kelly PJ, Wright GM, et al. Pulmonary metastasectomy for bone and soft tissue sarcoma in Australia: 114 patients from 1978 to 2008. Asia Pac J Clin Oncol 2012;8:292-302. [Crossref] [PubMed]
- Dossett LA, Toloza EM, Fontaine J, et al. Outcomes and clinical predictors of improved survival in a patients undergoing pulmonary metastasectomy for sarcoma. J Surg Oncol 2015;112:103-6. [Crossref] [PubMed]
- García Franco CE, Torre W, Tamura A, et al. Long-term results after resection for bone sarcoma pulmonary metastases. Eur J Cardiothorac Surg 2010;37:1205-8. [Crossref] [PubMed]
- Giuliano K, Sachs T, Montgomery E, et al. Survival following lung metastasectomy in soft tissue sarcomas. Thorac Cardiovasc Surg 2016;64:150-8. [Crossref] [PubMed]
- Harting MT, Blakely ML, Jaffe N, et al. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg 2006;41:194-9. [Crossref] [PubMed]
- Kim S, Ott HC, Wright CD, et al. Pulmonary resection of metastatic sarcoma: prognostic factors associated with improved outcomes. Ann Thorac Surg 2011;92:1780-6. [Crossref] [PubMed]
- Lin AY, Kotova S, Yanagawa J, et al. Risk stratification of patients undergoing pulmonary metastasectomy for soft tissue and bone sarcomas. J Thorac Cardiovasc Surg 2015;149:85-92. [Crossref] [PubMed]
- Mizuno T, Taniguchi T, Ishikawa Y, et al. Pulmonary metastasectomy for osteogenic and soft tissue sarcoma: who really benefits from surgical treatment? Eur J Cardiothorac Surg 2013;43:795-9. [Crossref] [PubMed]
- Okiror L, Peleki A, Moffat D, et al. Survival following Pulmonary Metastasectomy for Sarcoma. Thorac Cardiovasc Surg 2016;64:146-9. [Crossref] [PubMed]
- Pfannschmidt J, Klode J, Muley T, et al. Pulmonary metastasectomy in patients with soft tissue sarcomas: experiences in 50 patients. Thorac Cardiovasc Surg 2006;54:489-92. [Crossref] [PubMed]
- Rehders A, Hosch SB, Scheunemann P, et al. Benefit of surgical treatment of lung metastasis in soft tissue sarcoma. Arch Surg 2007;142:70-5. [Crossref] [PubMed]
- Reza J, Sammann A, Jin C, et al. Aggressive and minimally invasive surgery for pulmonary metastasis of sarcoma. J Thorac Cardiovasc Surg 2014;147:1193-200. [Crossref] [PubMed]
- Sardenberg RA, Figueiredo LP, Haddad FJ, et al. Pulmonary metastasectomy from soft tissue sarcomas. Clinics (Sao Paulo) 2010;65:871-6. [Crossref] [PubMed]
- Smith R, Pak Y, Kraybill W, et al. Factors associated with actual long-term survival following soft tissue sarcoma pulmonary metastasectomy. Eur J Surg Oncol 2009;35:356-61. [Crossref] [PubMed]
- Suri RM, Deschamps C, Cassivi SD, et al. Pulmonary resection for metastatic malignant fibrous histiocytoma: an analysis of prognostic factors. Ann Thorac Surg 2005;80:1847-52. [Crossref] [PubMed]
- Suzuki M, Iwata T, Ando S, et al. Predictors of long-term survival with pulmonary metastasectomy for osteosarcomas and soft tissue sarcomas. J Cardiovasc Surg (Torino) 2006;47:603-8. [PubMed]
- Van Geel AN, Pastorino U, Jauch KW, et al. Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer 1996;77:675-82. [Crossref] [PubMed]
- Welter S, Grabellus F, Bauer S, et al. Growth patterns of lung metastases from sarcoma: prognostic and surgical implications from histology. Interact Cardiovasc Thorac Surg 2012;15:612-7. [Crossref] [PubMed]
- Falk AT, Moureau-Zabotto L, Ouali M, et al. Effect on survival of local ablative treatment of metastases from sarcomas: a study of the French sarcoma group. Clin Oncol (R Coll Radiol) 2015;27:48-55. [Crossref] [PubMed]
- Hamaji M, Chen F, Miyamoto E, et al. Surgical and non-surgical management of repeat pulmonary metastasis from sarcoma following first pulmonary metastasectomy. Surg Today 2016;46:1296-300. [Crossref] [PubMed]
- Krüger M, Schmitto JD, Wiegmann B, et al. Optimal timing of pulmonary metastasectomy--is a delayed operation beneficial or counterproductive? Eur J Surg Oncol 2014;40:1049-55. [Crossref] [PubMed]
- Treasure T, Fiorentino F, Scarci M, et al. Pulmonary metastasectomy for sarcoma: a systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open 2012;2:e001736. [Crossref] [PubMed]
- Kang S, Kim HS, Kim S, et al. Post-metastasis survival in extremity soft tissue sarcoma: a recursive partitioning analysis of prognostic factors. Eur J Cancer 2014;50:1649-56. [PubMed]
- Porter GA, Cantor SB, Walsh GL, et al. Cost-effectiveness of pulmonary resection and systemic chemotherapy in the management of metastatic soft tissue sarcoma: a combined analysis from the University of Texas M. D. Anderson and Memorial Sloan-Kettering Cancer Centers. J Thorac Cardiovasc Surg 2004;127:1366-72. [Crossref] [PubMed]
- Thomford NR, Woolner L, Clagett O. The surgical treatment of metastatic tumours in the lung. J Thorac Cardiovasc Surg 1965;49:357-63. [PubMed]
- Pastorino U, Veronesi G, Landoni C, et al. Fluorodeoxyglucose positron emission tomography improves preoperative staging of resectable lung metastasis. J Thorac Cardiovasc Surg 2003;126:1906-10. [Crossref] [PubMed]
- Salati M, Brunelli A. Risk Stratification in Lung Resection. Curr Surg Rep 2016;4:37. [Crossref] [PubMed]
- McCormack PM, Ginsberg KB, Bains MS, et al. Accuracy of lung imaging in metastases with implications for the role of thoracoscopy. Ann Thorac Surg 1993;56:863-5. [Crossref] [PubMed]
- Macherey S, Doerr F, Heldwein M, et al. Is manual palpation of the lung necessary in patients undergoing pulmonary metastasectomy? Interact Cardiovasc Thorac Surg 2016;22:351-9. [Crossref] [PubMed]
- Kaifi JT, Gusani NJ, Deshaies I, et al. Indications and approach to surgical resection of lung metastases. J Surg Oncol 2010;102:187-95. [Crossref] [PubMed]
- Gossot D, Radu C, Girard P, et al. Resection of pulmonary metastases from sarcoma: can some patients benefit from a less invasive approach? Ann Thorac Surg 2009;87:238-43. [Crossref] [PubMed]
- Greenwood A, West D. Is a thoracotomy rather than thoracoscopic resection associated with improved survival after pulmonary metastasectomy? Interact Cardiovasc Thorac Surg 2013;17:720-4. [Crossref] [PubMed]
- Guerrini GP, Lo Faso F, Vagliasindi A, et al. The role of minimally invasive surgery in the treatment of lung metastases. J Invest Surg 2017;30:110-5. [Crossref] [PubMed]
- Pfannschmidt J, Hoffmann H, Schneider T, et al. Pulmonary metastasectomy for soft tissue sarcomas: is it justified? Recent Results Cancer Res 2009;179:321-36. [Crossref] [PubMed]
- Rusch VW. Pulmonary metastasectomy. Current indications. Chest 1995;107:322S-31S. [Crossref] [PubMed]
- Rolle A, Pereszlenyi A, Koch R, et al. Is surgery for multiple lung metastases reasonable? A total of 328 consecutive patients with multiple-laser metastasectomies with a new 1318-nm Nd:YAG laser. J Thorac Cardiovasc Surg 2006;131:1236-42. [Crossref] [PubMed]
- Linch M, Miah AB, Thway K, et al. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat Rev Clin Oncol 2014;11:187-202. [Crossref] [PubMed]
- Dancsok AR, Asleh-Aburaya K, Nielsen TO. Advances in sarcoma diagnostics and treatment. Oncotarget 2017;8:7068-93. [PubMed]
- Liebner DA. The indications and efficacy of conventional chemotherapy in primary and recurrent sarcoma. J Surg Oncol 2015;111:622-31. [Crossref] [PubMed]
- Canter RJ, Qin LX, Downey RJ, et al. Perioperative chemotherapy in patients undergoing pulmonary resection for metastatic soft-tissue sarcoma of the extremity: a retrospective analysis. Cancer 2007;110:2050-60. [Crossref] [PubMed]
- Bacci G, Rocca M, Salone M, et al. High grade osteosarcoma of the extremities with lung metastases at presentation: treatment with neoadjuvant chemotherapy and simultaneous resection of primary and metastatic lesions. J Surg Oncol 2008;98:415-20. [Crossref] [PubMed]
- Casey DL, Alektiar KM, Gerber NK, et al. Whole lung irradiation for adults with pulmonary metastases from Ewing sarcoma. Int J Radiat Oncol Biol Phys 2014;89:1069-75. [Crossref] [PubMed]
- Olivier T, Pop D, Chouiter Djebaili A, et al. Treating metastatic sarcomas locally: a paradoxe, a rationale, an evidence? Crit Rev Oncol Hematol 2015;95:62-77. [Crossref] [PubMed]
- Nakamura T, Matsumine A, Yamakado K, et al. Clinical significance of radiofrequency ablation and metastasectomy in elderly patients with lung metastases from musculoskeletal sarcomas. J Cancer Res Ther 2013;9:219-23. [Crossref] [PubMed]
- Palussière J, Italiano A, Descat E, et al. Sarcoma lung metastases treated with percutaneous radiofrequency ablation: results from 29 patients. Ann Surg Oncol 2011;18:3771-7. [Crossref] [PubMed]
- Koelblinger C, Strauss S, Gillams A. Outcome after radiofrequency ablation of sarcoma lung metastases. Cardiovasc Intervent Radiol 2014;37:147-53. [Crossref] [PubMed]
- Yellin A, Simansky DA, Paley M, et al. Hyperthermic pleural perfusion with cisplatin: early clinical experience. Cancer 2001;92:2197-203. [Crossref] [PubMed]
- den Hengst WA, Hendriks JM, Balduyck B, et al. Phase II multicenter clinical trial of pulmonary metastasectomy and isolated lung perfusion with melphalan in patients with resectable lung metastases. J Thorac Oncol 2014;9:1547-53. [Crossref] [PubMed]
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. WHO Classification of Tumours of Soft Tissue and Bone. 4th editon. Lyon: IARC Press, 2013.
- Burt BM, Ocejo S, Mery CM, et al. Repeated and aggressive pulmonary resections for leiomyosarcoma metastases extends survival. Ann Thorac Surg 2011;92:1202-7. [Crossref] [PubMed]
- Gadd MA, Casper ES, Woodruff JM, et al. Development and treatment of pulmonary metastases in adult patients with extremity soft tissue sarcoma. Ann Surg 1993;218:705-12. [Crossref] [PubMed]
- Ueda T, Uchida A, Kodama K, et al. Aggressive pulmonary metastasectomy for soft tissue sarcomas. Cancer 1993;72:1919-25. [Crossref] [PubMed]
- Casson AG, Putnam JB, Natarajan G, et al. Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer 1992;69:662-8. [Crossref] [PubMed]
- Welter S, Grabellus F, Bauer S, et al. Growth patterns of lung metastases from sarcomas. Virchows Arch 2011;459:213-9. [Crossref] [PubMed]
- Nicolas M, Moran CA, Suster S. Pulmonary metastasis from liposarcoma. A clinicopathologic and immunohistochemical study of 24 cases. Am J Clin Pathol 2005;123:265-75. [Crossref] [PubMed]
- Stephens EH, Blackmon SH, Correa AM, et al. Progression after chemotherapy is a novel predictor of poor outcomes after pulmonary metastasectomy in sarcoma patients. J Am Coll Surg 2011;212:821-6. [Crossref] [PubMed]
- Avital I, DeMatteo R. Combined resection of liver and lung metastases for colorectal cancer. Thorac Surg Clin 2006;16:145-55. [Crossref] [PubMed]
- Ohnstad HO, Bruland OS, Taksdal I, et al. Response to preoperative chemotherapy in patients undergoing resection of pulmonary metastasis from soft tissue sarcoma—a predictor of outcome? Acta Oncol 2014;53:1180-7. [Crossref] [PubMed]
- Iwata S, Yonemoto T, Iizasa T, et al. Oligo-recurrence of osteosarcoma patients: treatment strategies for pulmonary metastases. Ann Surg Oncol 2015;22:S1332-8. [Crossref] [PubMed]
- Liebl LS, Elson F, Quaas A, et al. Value of repeat resection for survival in pulmonary metastases from soft tissue sarcoma. Anticancer Res 2007;27:2897-902. [PubMed]
- Chen F, Miyahara R, Bando T, et al. Repeat resection of pulmonary metastasis is beneficial for patients with osteosarcoma of the extremities. Interact Cardiovasc Thorac Surg 2009;9:649-53. [Crossref] [PubMed]
- Weiser MR, Downey RJ, Leung DH, et al. Repeat resection of pulmonary metastases in patients with soft-tissue sarcoma. J Am Coll Surg 2000;191:184-90. [Crossref] [PubMed]


