Should the cut-off value of D-dimer be elevated to exclude pulmonary embolism in acute exacerbation of COPD?
Introduction
Venous thromboembolism (VTE), specifically deep vein thrombosis (DVT) and pulmonary embolism (PE) is an important cause of morbidity and mortality (1). Clinical suspicion is important in diagnosing PE. The presence of unexplained respiratory symptoms, such as dyspnea and chest pain, in a patient with VTE risk factors implies PE. It is neither possible nor cost-effective to perform further diagnostic procedures, such as thorax computed tomography angiography (CTA) and venous Doppler ultrasonography (US), in each clinically suspect patient. PE was detected in only 25% of suspected PE patients who were evaluated by further diagnostic procedures (2). The use of D-dimer, which is a simple and comparatively noninvasive test, excludes PE without further imaging procedures (3). D-dimer is a manifestation of endogen fibrinolytic activity (4). The concentration of D-dimer in plasma increases in all physiological and pathological cases in which fibrin has formed and then is degraded by plasmin. Chronic inflammatory diseases occur under clinical conditions that may increase D-dimer levels (5). Recent studies have shown that systemic inflammation is associated with chronic obstructive pulmonary disease (COPD) (6). The presence of elevated prominent systemic inflammatory markers, such as C-reactive protein and tumor necrosis factor alpha, in the plasma of COPD patients indicated permanent systemic inflammation in these patients (7). The prevalence of PE is increased in the COPD patients who were in the period of acute exacerbation (8,9). Moreover, COPD has recently been defined as an independent risk factor for PE (10). There is conflicting data concerning the D-dimer levels in COPD patients. Hartmann et al. have proposed that D-dimer has similar accuracy in COPD patients with or without PE (11). Silva et al. observed that D-dimer levels did not differ in stable COPD patients compared to control subjects (12). However, several case-control studies have suggested that the presence of the hypercoagulable state in COPD patients was associated with higher D-dimer levels than in control subjects (13,14). It was also shown that D-dimer may increase in patients with COPD exacerbation irrespective of presence of VTE (15,16).
The aim of this study was to evaluate the D-dimer levels in COPD patients who were in acute exacerbation with and without PE and to attempt to define a new cut-off value for D-dimer to exclude the diagnosis of PE in COPD patients who are in the exacerbation period.
Methods
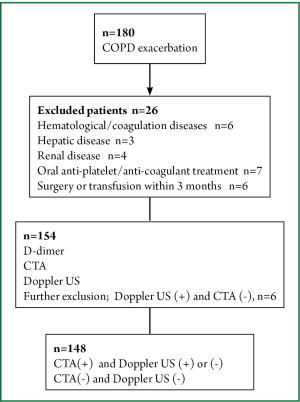
This cross-sectional study was performed between June 2012 and January 2013 at Ufuk University in Ankara, the capital city of Turkey. The study protocol was approved by the Ufuk University Ethical Committee and informed consent was given by all patients who participated in the study. Patients who were admitted to the emergency department because of COPD exacerbation were consecutively enrolled to the study. The COPD diagnosis was based on spirometry that was previously performed during the stable period of the disease using Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (17). Venous blood samples were taken upon admission to measure the D-dimer levels. All patients underwent CTA and venous Doppler US of the lower extremities within 4 hours of admission to the emergency department without considering the patients’ D-dimer levels. Patients with hematological diseases, coagulation disorders, hepatic or renal diseases, under oral anti-platelet or oral anti-coagulant therapy, known malignancies or collagen vascular diseases at admission were excluded, as were patients who had undergone surgery or transfusion in the previous 3 months. The D-dimer levels were measured with the Tina-quant® D-dimer Test system (Boehringer, Mannheim) which is a particle enhanced immunoturbidimetric assay. All CTA studies were analyzed on a dedicated workstation (Advanced Workstation 4.0, GE Healthcare, Milwaukee, Wisconsin, USA). PE was diagnosed when an intraluminal filling defect surrounded by intravascular contrast or total occlusion of the pulmonary arterial lumen were detected at any level of the pulmonary arteries. Doppler US of the deep veins of lower extremities was performed with a standard method using ultrasound equipment (LOGIQ 7®, GE Healthcare, Milwaukee, Wisconsin, USA) with a broad-bandwidth linear array transducer (model 10 L; bandwidth, 6-10 MHz) to investigate the presence or absence of intravenous thrombus. Patients who had DVT on Doppler US, but did not have thrombus on CTA were excluded from the study. The study design is shown in Figure 1.
Statistical analysis
Data were summarized as the mean ± standard deviation and median (minimum-maximum) for continuous variables and frequencies (percentiles) for the categorical variables. The Student’s t-test or Mann-Whitney U-test was used for group comparisons, depending on the distributional properties of the data. The Chi-squared test was used for proportions. The receiver operating characteristics (ROC) curve was obtained for the various D-dimer cut-off values, a predictor of PE. Youden’s index was calculated (YI = sensitivity + specificity –1) for each coordinate point of the ROC curve to determine the cut-off value, which has the maximum sensitivity and specificity pair. All analyses were performed using IBM SPSS Statistics for Windows, Version 20.0, and P value <0.05 was considered as statistically significant.
Results
A total of 148 patients were enrolled in the study. The mean participant age was 73.3±8.5 years [108 (73%) males]. The patient distribution according to the GOLD stages (GOLD 1, 2, 3, 4) was as follows: 0%, 43.92%, 25.68%, and 30.4%, respectively. The mean D-dimer level of the patients was 1.56±2.18 pg/mL. Thrombus was detected on CTA in 37.8% of patients. All patients who had thrombus on CTA also had thrombus of the lower extremities on Doppler US. The Doppler US of the patients without PE were interpreted as normal.
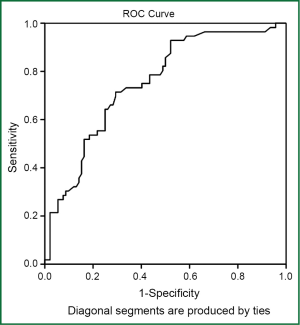
The age and gender distribution of the patients with and without PE were not significantly different (P>0.05). Fifty-three patients (36%) without PE had D-dimer levels that were higher than normal (>0.5 pg/mL). The D-dimer levels of the COPD patients with PE were significantly higher than the patients without PE (P<0.001). The age, gender distribution and D-dimer levels of the COPD patients with and without PE are shown in Table 1. The D-dimer cut-off value in diagnosing PE in the COPD patients was 0.95 pg/mL. The area under the ROC curve was found to be 0.752±0.040 (95% CI: 0.672-0.831) (P<0.001) (Figure 2).

Full Table
Discussion
This study showed that the D-dimer concentration of COPD patients in the exacerbation period may be higher than normal, although they do not have PE. In this study, the D-dimer cut-off level was 0.95 pg/mL (sensitivity 70%, specificity 71%) for the exclusion of PE in patients with COPD exacerbation.
COPD patients have a higher risk of developing PE, most likely, because of immobility, polycythemia, advanced age and associating systemic inflammation (6,11). It was recently reported that the prevalence of PE increased among COPD patients (8,9). Günen et al. proposed that all COPD patients hospitalized because of acute exacerbation should be evaluated for the presence of VTE (9). However, it is difficult to differentiate other causes of COPD exacerbation from PE because of the common presenting signs and symptoms. Therefore, D-dimer testing plays a more critical role in excluding the PE diagnosis in these patients. In recent years, higher clinical suspicion of PE due to increased awareness of disease by clinicians using diagnostic algorithms including D-dimer causes harmful consequences, such as radiation exposure, and nephrotoxicity because of contrast material used for CTA. Further imaging techniques, such as thorax CTA, also must be interpreted by an expert radiologist: it is not cost-effective to evaluate all suspected PE patients using these techniques.
Sohne et al. showed that the combination of low clinical probability with normal level of D-dimer offered similar safety in excluding of PE in COPD patients (18). Akgün et al. showed that patients with COPD exacerbation and VTE had higher D-dimer levels than COPD patients who without VTE (15). However, there is no evidence concerning the accuracy of D-dimer levels in diagnosing PE in COPD patients. COPD itself may cause false positivity in D-dimer measurement. Contradictory results have been described about the D-dimer levels in patients with stable COPD (12-14).
D-dimer is a fibrin degradation product and shows endogenous fibrinolytic activity. It has been reported that the presence of hypercoagulable state in COPD patients is associated with higher D-dimer levels than healthy subjects (13,14). The hypercoagulable state, which is associated with COPD, may increase during the exacerbation period. In this study, the Tina-quant® method was used to measure D-dimer levels. The negative predictive value for D-dimer in the diagnosis of PE is 99% with this method, and the cut-off value is 0.5 pg/mL (19). In our study, the D-dimer level was higher than 0.5 pg/mL in 36% of the COPD patients, although they did not have PE. Therefore, the use of this cut-off value may lead to overuse of further diagnostic procedures. Raviv et al. retrospectively evaluated the possibility of using a higher D-dimer value in all patients with suspected PE who are examined in emergency departments, without considering the presence of COPD. They found the cut-off value to be 900 ng/mL with a sensitivity of 94.4% (20). To our knowledge, our study was the first study evaluating the cut-off value of D-dimer to exclude PE in COPD patients who are in the exacerbation period. Hartmann et al. suggested that presence of COPD does not affect the diagnostic performance of D-dimer (11). However, they evaluated D-dimer values of patients with and without COPD as “normal” or “abnormal”. We compared the exact D-dimer values of COPD patients with and without PE.
Aging increase the levels of D-dimer. In a retrospective study that included suspected PE patients who were evaluated in the emergency department, the authors suggested raising the cut-off value of D-dimer in the evaluation of PE based on the patient age (20). Recent studies also suggested that using an age-adjusted cut-off value, safely excludes DVT in elderly patients (21,22). In our study, the mean ages of the COPD patients with and without PE were not significantly different. Therefore, the effect of aging, which may bias the study results was minimized.
This study had some limitations. The small number of COPD patients may have limited the ability to define the exact cut-off value of D-dimer necessary to exclude PE. This was a cross-sectional study, prospective studies in which repeated D-dimer measurements will be performed in patients with COPD exacerbation with and without PE, may be helpful for designating the kinetics of D-dimer in these patients. In this study, the COPD patients who had co-morbidities which may cause elevation of D-dimer levels beyond the effect of COPD and PE, such as malignancies, collagen vascular diseases, were excluded. So that, the study population was not representative for all COPD patients. This was also a limitation of the study. CTA was used to show PE in this study. This imaging procedure may not detect thrombi that are located subsegmental level of the pulmonary arteries. Ventilation/perfusion scan may be more helpful at this level.
In conclusion, the D-dimer cut-off value that is used to exclude PE in patients with acute exacerbation of COPD should be reevaluated to prevent the excessive use of further diagnostic procedures, such as CTA. In this study, the proposed D-dimer cut-off level for excluding PE in patients with COPD exacerbation was 0.95 pg/mL. Larger studies are needed to define the exact cut-off level of D-dimer in COPD patients who are in the exacerbation period.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- White RH. The epidemiology of venous thromboembolism. Circulation 2003;107:14-8. [PubMed]
- Le Gal G, Bounameaux H. Diagnosing pulmonary embolism: running after the decreasing prevalence of cases among suspected patients. J Thromb Haemost 2004;2:1244-6. [PubMed]
- Righini M, Perrier A, De Moerloose P, et al. D-dimer for venous thromboembolism diagnosis: 20 years later. J Thromb Haemost 2008;6:1059-71. [PubMed]
- Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost 2000;83:416-20. [PubMed]
- Prisco D, Grifoni E. The role of D-dimer testing in patients with suspected venous thromboembolism. Semin Thromb Hemost 2009;35:50-9. [PubMed]
- Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009;33:1165-85. [PubMed]
- Gan WQ, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004;59:574-80. [PubMed]
- Rizkallah J, Man SF, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest 2009;135:786-93. [PubMed]
- Gunen H, Gulbas G, In E, et al. Venous thromboemboli and exacerbations of COPD. Eur Respir J 2010;35:1243-8. [PubMed]
- Poulsen SH, Noer I, Moller JE, et al. Clinical outcome of patients with suspected pulmonary embolism. A follow-up study of 588 consecutive patients. J Intern Med 2001;250:137-43. [PubMed]
- Hartmann IJ, Hagen PJ, Melissant CF, et al. Diagnosing acute pulmonary embolism: effect of chronic obstructive pulmonary disease on the performance of D-dimer testing, ventilation/perfusion scintigraphy, spiral computed tomographic angiography, and conventional angiography. Am J Respir Crit Care Med 2000;162:2232-7. [PubMed]
- Silva DR, Coelho AC, Gazzana MB, et al. D-dimer levels in stable COPD patients: a case-control study. COPD 2012;9:426-31. [PubMed]
- Arregui MA, Ezquerra KL, López FC, et al. Hypercoagulability state and endotelial injury in stable chronic obstructive pulmonary disease patients. An Sist Sanit Navar 2010;33:43-50. [PubMed]
- Alessandri C, Basili S, Violi F, et al. Hypercoagulability state in patients with chronic obstructive pulmonary disease. Chronic Obstructive Bronchitis and Haemostasis Group. Thromb Haemost 1994;72:343-6. [PubMed]
- Akgun M, Meral M, Onbas O, et al. Comparison of clinical characteristics and outcomes of patients with COPD exacerbation with or without venous thromboembolism. Respiration 2006;73:428-33. [PubMed]
- Karwat K, Kościuch J, Chazan R. Is microembolism present and is it important element of COPD exacerbation? Pol Merkur Lekarski 2005;18:385-8. [PubMed]
- As recommended by the global strategy for the diagnosis, management and prevention of COPD, global initiative for chronic obstructive lung disease (GOLD) 2011. Available online: http://www.goldcopd.org/, Accessed April 4, 2013.
- Sohne M, Kruip MJ, Nijkeuter M, et al. Accuracy of clinical decision rule, D-dimer and spiral computed tomography in patients with malignancy, previous venous thromboembolism, COPD or heart failure and in older patients with suspected pulmonary embolism. J Thromb Haemost 2006;4:1042-6. [PubMed]
- Nomura H, Wada H, Mizuno T, et al. Negative predictive value of D-dimer for diagnosis of venous thromboembolism. Int J Hematol 2008;87:250-5. [PubMed]
- Raviv B, Israelit SH. Shifting up cutoff value of d-dimer in the evaluation of pulmonary embolism: a viable option? Possible risks and benefits. Emerg Med Int 2012;2012:517375.
- Douma RA, Tan M, Schutgens RE, et al. Using an age-dependent D-dimer cut-off value increases the number of older patients in whom deep vein thrombosis can be safely excluded. Haematologica 2012;97:1507-13. [PubMed]
- Schouten HJ, Koek HL, Oudega R, et al. Validation of two age dependent D-dimer cut-off values for exclusion of deep vein thrombosis in suspected elderly patients in primary care: retrospective, cross sectional, diagnostic analysis. BMJ 2012;344:e2985. [PubMed]