Hybrid and total minimally invasive esophagectomy: how I do it
Introduction
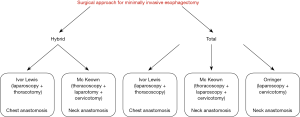
Esophageal cancer ranks eight among the most common malignancies and the incidence is rapidly increasing in the Western world at a rate greater than any other type of solid tumor. Esophagectomy represents the current standard of care for patients with localized esophageal carcinoma staged as T1sm/N+ or higher. A protocol of multidisciplinary treatment is generally recommended in patients with locally advanced tumors, especially in those with squamous-cell carcinoma (1). Esophagectomy is a complex surgical procedure that requires a two or three-field access depending on preoperative clinical staging, location and histology of the tumor, comorbidity, and patient’s anatomy and physiological status. Although the current postoperative mortality has decreased to less than 5% in high-volume centers, complications related to anastomotic and respiratory failure are still significant and appear to be independent of the surgical approach and the anastomotic technique. The overall morbidity of the operation has not been significantly reduced over the past few decades, even with the trans-hiatal approach, indicating that the pathogenesis of complications associated with esophagectomy is multifactorial and not entirely dependent on the surgical access and length of the skin incisions. Minimally invasive surgery was introduced in the 90s’ with the aim to decrease the rate of respiratory complications associated with the thoracotomy approach. A number of hybrid and total minimally invasive surgical approaches have been developed and are currently applied in several centers worldwide. The most commonly performed hybrid procedure is a modification of the classic 2-stage Ivor Lewis operation, in which the laparotomy is replaced by laparoscopy for gastric conduit preparation and celiac lymphadenectomy. The total minimally invasive esophagectomy includes two techniques: the 3-stage thoraco-laparoscopic esophagectomy, a modification of the classic McKeown operation in which right thoracotomy and laparotomy are replaced by thoracoscopy and laparoscopy, and the minimally invasive trans-hiatal esophagectomy, a modification of the classic 2-stage trans-hiatal esophagectomy, in which the laparotomy is replaced by laparoscopy (Figure 1).
Patient selection and surgical strategy
Patients with esophageal carcinoma require an extensive preoperative staging, including CT scan and/or endoscopic ultrasonography, flexible bronchoscopy, and PET scan when appropriate, to exclude locally advanced or metastatic disease. Assessment of the functional, nutritional and comorbidity status is necessary before considering the patient for immediate surgery or for a multimodality treatment plan determined in a multidisciplinary oncological team meeting (2). Preparation for surgery should include abstinence from smoking, daily walking activity, use of an incentive spirometer, and a 1-week preoperative enteral nutritional support in patients with long-lasting dysphagia, significant weight loss, and a pre-frail or frail phenotype.
Preoperative staging and tumor characteristics influence the choice of the surgical strategy, i.e., a 2-stage or a 3-stage procedure. In some circumstances, starting with laparoscopy or thoracoscopy may be useful to provide the ultimate staging. Initial laparoscopic approach for gastric conduit preparation, as part of a hybrid or total minimally invasive Ivor Lewis operation, is feasible in the majority of patients with esophageal adenocarcinoma (3). When tumor resectability is doubtful, as in patients with squamous-cell cancer of the middle-upper thoracic esophagus after neoadjuvant therapy, a primary thoracoscopy/thoracotomy as part of a hybrid or total minimally invasive McKeown approach may be more appropriate. However, to avoid surprises during the subsequent phase of the operation, the presence of peritoneal carcinosis, tumor involvement of the gastric fundus, liver metastases, and important comorbidities such as liver cirrhosis with portal hypertension, should be ruled out beforehand (4). Anatomical factors such as a short “bull” neck may suggest to avoid a neck anastomosis if not strictly necessary. In some circumstances, following an initial thoracic approach, a decision can be made to return into the chest for the anastomosis after the gastric conduit has been prepared by laparoscopy.
From a surgical and oncological standpoint, the ideal candidate for a primary thoracic approach is a patient with a clinically staged T1–3 squamous-cell carcinoma of the upper/middle thoracic esophagus. Initial thoracoscopy may be an option also in patients with type I esophageal adenocarcinoma, especially in those with extra-long Barrett’s esophagus. A narrow upper mediastinum and the presence of spine abnormalities such as dorsal kyphosis, scoliosis, and vertebral osteophytosis, may represent a relative contraindication to the thoracoscopic approach in the prone position because of the technical difficulties to access the esophagus hidden by the vertebral bodies, and the altered anatomical relationships with the aorta and the tracheobronchial tree (5). In these circumstances, the semi-prone position has some advantages over the prone position.
Surgical procedures
Hybrid Ivor Lewis esophagectomy
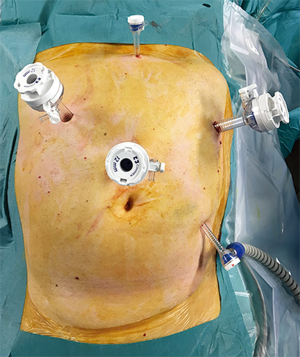
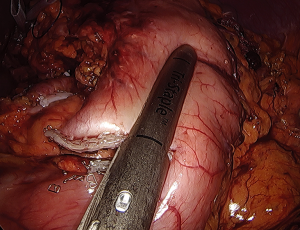
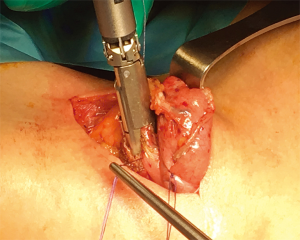
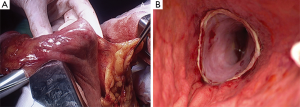
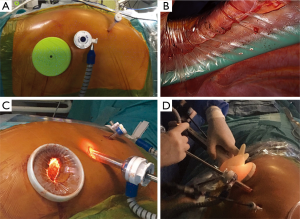
The operation consists of a 2-stage approach (laparoscopy + right thoracotomy). The laparoscopic phase is performed with the patient placed in a reverse Trendelenburg position. After induction of pneumoperitoneum with the Veress needle, five 5–12 mm ports are placed (Figure 2). Dissection is performed using the hook cautery and ultrasonic scissors beginning with division of the gastrohepatic ligament. The stomach is mobilized by dividing the left gastric vessels (Figure 3) and short gastrics, and separating the right gastroepiploic arcade from the gastrocolic omentum. A standard D2-lymphadenectomy is performed. A 4-cm wide gastric conduit is constructed by sequential firings of 45–60 mm Endo-GIA® (Medtronic) cartridges parallel to the greater curvature. The first 45 mm cartridge is applied across the lesser curve cranial to the third branch of the right gastric artery and is directed almost at right angle toward the greater curve; special care is required to maintain a consistent width of the stomach and to avoid spiralization of the gastric tube during application of the subsequent cartridges (Figure 4). Interrupted 4-0 PDS stitches are applied at the intersection of the staple lines. To prevent diaphragmatic hernia, a posterior suture of the crura is placed and left temporarily untied into the lower mediastinum. A 15 Fr Blake® drain (Ethicon) is placed in the mediastinum through the upper abdominal port and then through the hiatus. For the thoracotomy phase of the operation, the right lung is excluded using a left double-lumen tube or an endobronchial blocker under fiberoptic bronchoscopic guidance, and the patients is turned to the left lateral position with a roll at the level of the tip of the scapula. A right postero-lateral incision sparing the serratus muscle is performed in the fifth intercostal space and the lung is retracted medially. The arch of the azygos vein is divided with Hemolock clips and the thoracic duct is selectively ligated above the diaphragm. A standard infracarinal lymphadenectomy is performed in patients with adenocarcinoma, whereas paratracheal nodes are routinely removed only in squamous-cell carcinoma. The esophago-gastric anastomosis is performed at the apex of the right chest using a 25 mm EEA® stapler (Medtronic) (Figure 5). The pleural cavity is drained by the trans-abdominal Blake drain.
Total minimally invasive Ivor Lewis esophagectomy
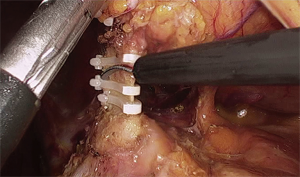
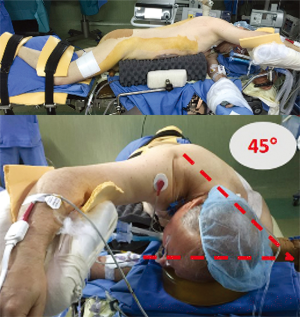
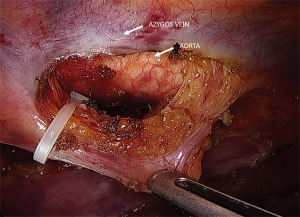
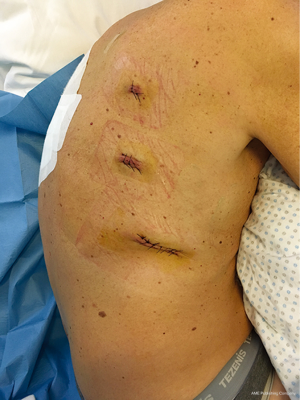
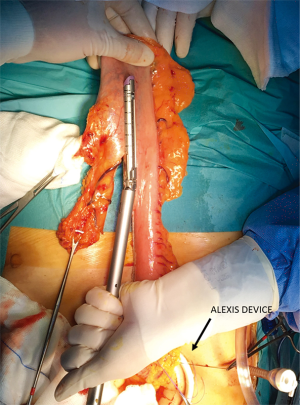
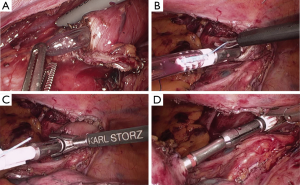
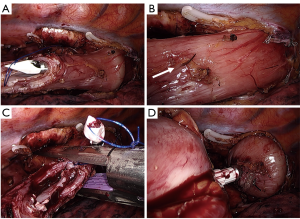
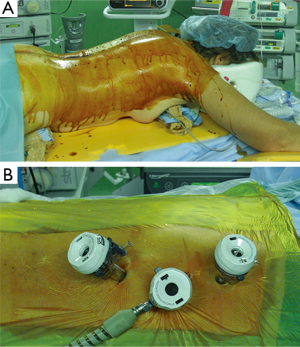
The laparoscopic stage of the procedure, including lymphadenectomy and gastric mobilization, is similar to that of the hybrid operation. Interrupted 4-0 PDS stitches applied at the intersection of the staple lines along the lesser curve and the apex of greater curve are of utmost importance to help retrieval and proper orientation of the gastric tube from the chest cavity during the thoracoscopic stage. The patient is placed in the semi-prone position with the right arm positioned on a support device and the forearm flexed to improve abduction of the scapula. The chest is stabilized on the operative table using bean bag and side supports to allow rotation in a more lateral decubitus position (Figure 6). This is helpful to aid mediastinal exposure in patients with a protruding spine or to expedite the switch to thoracotomy if necessary. After exclusion of the right lung and induction of pneumothorax with a Veress needle in the posterior axillary line, three trocars (two 12 mm and one 5 mm) are placed in the fourth, sixth and eighth intercostal space. The arch of the azygos vein is divided using Hemolock clips or a vascular EndoGIA stapler. Incision of the mediastinal pleura is performed on both sides of the esophagus, and the dissection preferably starts between the vagal trunk and the right main bronchus. This allows en-bloc lymphadenectomy of the carina with nerve preservation in most circumstances. The esophagus is then mobilized up to the level of the diaphragm and the inferior pulmonary ligament is divided. The thoracic duct is identified and ligated with a single Hemolock® clip (Figure 7). The 25 mm anvil of a circular stapler (Orvil®, Medtronic) can be inserted trans-orally and retrieved through a small hole close to the stapled line of the esophageal stump (Figure 8). Another option is to insert the anvil attached to a 2-0 polypropylene suture in the esophageal lumen through an esophagotomy; the needle is then retrieved by reverse puncture of the anterior esophageal wall and, once the anvil rod is out, the esophagus is divided with a linear stapler (Figure 9). At this point, the incision corresponding to the lowermost trocar is enlarged to 5 cm in length toward the anterior axillary line and a wound retractor device (Alexis®, Applied Medical) is inserted. The distal end of the circular stapler, enveloped in a surgical glove through a small cut in the middle finger, is inserted through the mini-thoracotomy wound with minimal dilatation of the intercostal space. The surgical glove, by adhering to the borders of the wound retractor, allows to maintain the pneumothorax during the anastomosis. The stapler is then advanced through a small gastrotomy on the lesser curve side, perforates the tip of the greater curve and engages the anvil (Figure 10). After checking the donoughts, transection of the excess stomach with a linear stapler is performed. The specimen is placed in an endobag and extracted from the chest cavity through the minithoracotomy (Figure 11).
Hybrid and total minimally invasive McKeown esophagectomy
The operation consists of a 3-stage esophagectomy with cervical anastomosis. Cuschieri first reported a thoracoscopic esophageal mobilization with the patient in the prone position in 1992 (6). The thoracoscopic approach was later adopted by other surgeons with the patient in the left-lateral decubitus (7). The prone position was revisited and popularized by Palanivelu in 2006 (8). A number of reports have subsequently evaluated this procedure and it appears that the prone position has some advantages over the left-lateral decubitus position (9). Thoracoscopic esophagectomy in the prone position is performed after induction of anesthesia with a single-lumen tracheal tube. The patient is then turned to the prone position, and chest and pelvic supports to leave the abdomen free for breathing excursions. A special headrest support with an integrated mirror (Disposa-View®) allows the anesthesiologist to check the position of the tube. Collapse of the right lung is obtained by CO2 insufflation through a Veress needle. Three ports are placed in the fourth, sixth and eighth intercostal space (Figure 12). Gas insufflation pressure is maintained at 8 mmHg. After incision of the mediastinal pleura, the arch of the azygos vein is divided with Hemolock clips. Esophageal dissection, mediastinal lymphadenectomy and transection of the upper thoracic esophagus are then performed following the same principles described before. A perianastomotic Blake drain is placed before turning patient to the supine position. During the laparoscopic phase of the operation, the right gastroepiploic arcade is separated from the gastrocolic omentum. The short gastric vessels and the left gastric artery and vein are divided. The celiac nodes are excised along the common hepatic and splenic vessels. At this point, the hiatus is widely opened and the previously transected esophagus is retrieved in the abdominal cavity. A 4-cm wide gastric conduit is fashioned either extra-corporeally, through an upper midline 5 cm minilaparotomy, or intra-corporeally, and then gently pulled through the posterior mediastinum under laparoscopic control up to the left neck incision. A semi-mechanical esophago-gastric anastomosis is performed using a 45 mm Endo-GIA stapler (Figure 13). Use of the semiprone position provides the same benefits of the prone position in terms of ergonomics and respiratory parameters, the difference being that switch to thoracotomy is feasible by tilting the table without changing patient position.
Hybrid and total minimally invasive trans-hiatal esophagectomy
The operation can be performed by a single surgical team or two teams. The patient is placed supine on the operative table, the neck extended toward the left side. A standard 5-port laparoscopic set-up is used. Celiac lymphadenectomy and gastric mobilization are performed as described before. Dissection of the esophagus and paraesophageal lymph nodes is performed through the hiatus up to the level of the inferior pulmonary vein. A left cervical incision on the anterior border of the sternocleidomastoid muscle is performed to dissect the proximal esophagus. This is then divided and the distal stump is attached to a Levine tube. The inverted esophagus is progressively retrieved in the abdominal cavity under laparoscopic assistance. The gastric tubulization can be performed extracorporeally through a 5 cm upper midline minilaparotomy protected with an Alexis wound retractor (Figure 14). The gastric tube is then stitched to a 28 Argyle tube and gently retrieved from the neck under laparoscopic assistance. An alternative technical option is to prepare the gastric tube intracorporeally, leave it attached to the gastroesophageal junction, and gently retrieve the mobilized esophagus and gastric tube from the neck under laparoscopic assistance. Finally, a stapled side-to-side semimechanical esophago-gastric anastomosis is created in the neck.
Perioperative management
A standardized clinical pathway protocol is followed in all patients undergoing minimally invasive esophagectomy. An epidural catheter is generally used for postoperative analgesia. In selected patients with contraindications to epidural analgesia, a serratus anterior plane block is performed (10). Antibiotic prophylaxis with Cefazolin is given. An arterial line is routinely placed for blood pressure monitoring. Two large-bore intravenous lines are adequate, and a central line is rarely needed. Intraoperative normothermia is maintained using a warm air blanket and avoiding use of vasoconstrictor agents. The volume of fluid administration is restricted to a maximum of 2 L. Patients are usually extubated in the operating room and transferred to the intensive care unit. Pain management consists of levobupivacaine through the epidural catheter combined with intravenous paracetamol as needed. Nasogastric aspiration is maintained during the first 48 hours. Patients are allowed to ambulate and to begin pulmonary physiotherapy with an incentive spirometer on postoperative day one. Water sips and fruit jelly are allowed on day 3, and then the diet is gradually progressed. A gastrografin swallow study is routinely performed on day 5 or 6. Patients are discharged from the hospital when the following criteria are met: no laboratory or clinical evidence of infection, ability to fully ambulate without assistance, no major analgesic requirements, oral diet well tolerated without significant gastrointestinal discomfort.
Discussion
Incremental steps of innovation in esophageal surgery have resulted in reduced postoperative mortality and improved oncological outcomes. With the advent of minimally invasive surgery, pros and cons of 2-stage and 3-stage procedures have been critically revisited. A recent survey has found a worldwide increase in the adoption of minimally invasive esophagectomy and a rise of high-volume centers. However, differences still exist regarding the extent of nodal dissection and site of anastomosis. The most favoured approach remains the minimally invasive McKeown operation followed by the minimally invasive Ivor Lewis operation. The preference for the transhiatal esophagectomy has decreased from 26% in 2007 to 15% in 2014 (11).
Today, the minimally invasive esophagectomy can be performed with minimal blood loss, controlled pain, and reduced intensive care unit stay and pulmonary complications compared to the open procedure. Short-term outcomes of minimally invasive esophagectomy have proven at least equivalent to the open approach in meta-analyses (12,13), a large administrative national database (14), and a randomized clinical trial (15). In addition, minimally invasive esophagectomy has been associated with a rapid restoration of health-related quality of life (16,17).
Both the hybrid and the total minimally invasive 2-stage and 3-stage esophagectomy are included in the definition of minimally invasive esophagectomy. The hybrid Ivor Lewis operation has been shown to have a reasonable learning curve and to be reproducible (18-20). Whether the laparoscopic component of the operation will decrease the major complication rate in esophageal cancer surgery has not been completely clarified yet. A French nationwide study has shown that laparoscopic gastric mobilization as a part of the hybrid esophagectomy significantly reduced postoperative mortality both at 30 and 90 days (21). Preliminary results of the Miro trial show a reduction in severe complications and major pulmonary complications without a negative impact on oncological outcomes and a trend toward better survival (22). On the other hand, Briez and colleagues (23) found that the hybrid Ivor Lewis approach was an independent factor protecting against major pulmonary complications when compared to open surgery. This may be related to the fact that laparoscopy can mitigate the mechanical and immunological stress associated with one lung ventilation and left lateral decubitus position. A total minimally invasive Ivor Lewis approach with thoracoscopic anastomosis is now preferred, but its use is not widespread due to the difficulties in performing the anastomosis. The increased prevalence of adenocarcinoma justifies the efforts to adopt the total minimally invasive Ivor Lewis approach in the future despite the superior technical difficulties and the steep learning curve. It has been estimated that a reasonable learning curve for the total minimally invasive Ivor Lewis esophagectomy would require 35–40 patients to achieve improved results (24). The technique of intrathoracic anastomosis needs to be standardized and more data are needed to assess the efficacy of the various proposed methods. The results of the intrathoracic stapled anastomosis appear encouraging, but no single technique has proven superior to the others (25). Pooled data including 282 patients from 6 European centers showed a 15.2% incidence of anastomotic leakage. Only 13 patients (4.6%) had pleural empyema requiring thoracotomy for decortication, and the 30-day and in-hospital mortality rate was 2.1%. A R-0 resection was obtained in 92.5% of patients (26).
Concerning the 3-stage McKeown esophagectomy, the adoption of the prone and semiprone position has represented a major advance in the performance of the thoracoscopic phase of the operation. This approach has allowed 2-lung ventilation, further reduction of operative trauma, and improved surgical ergonomics compared to the left lateral decubitus (4,9,27). The TIME trial has provided evidence that thoracoscopic prone esophagectomy is associated with a lower incidence of in-hospital pulmonary infections and a shorter hospital stay compared to the open approach (15). More recently, there has been a shift in western countries from the 3-stage operation in favor of the total minimally invasive Ivor Lewis approach. This has been attributed to an increased referral of patients with esophageal adenocarcinoma and an effort to reduce the morbidity associated with recurrent nerve injuries (28). The 3-stage approach requires a cervical anastomosis and is also more time-consuming. Although the mean surgical time for the thoracoscopic esophagectomy is about one hour and is significantly reduced in the prone position compared to the left lateral decubitus (9), prone positioning and then repositioning requires extra-time in the operating room and at least 6 staff members including the anesthesiologist. Avoiding the thoracotomy incision and releasing the right chest and abdomen from compression during the prone position provides better oxygen delivery, decreases the pulmonary shunt, and improves the ventilation perfusion match (27). This could in turn decrease the incidence of respiratory (15) and anastomotic (29) complications. In addition, preservation of two-lung ventilation can significantly reduce the ischemia-reperfusion injury and the oxidative stress (30). In the future, the role of robot-assisted esophagectomy may increase due to the benefits of a stable three-dimensional image and more precise dissection by avoiding the fulcrum effect of the instruments at the ribs and their parallel approach at the thoracic inlet and toward the diaphragm. In addition, robot-assisted surgery may enable to perform a safer manual anastomosis. A randomized trial comparing open and robot-assisted esophagectomy is ongoing (31). Common reservations about the safety of 3-stage esophagectomy (recurrent nerve injury, gastric conduit necrosis and twisting, “catastrophic” anastomotic leakage) have not been confirmed in our experience. The semimechanical anastomosis in the neck was as safe as a circular anastomosis at the apex of the chest (4), confirming the fact that neck and chest anastomoses are equally safe when performed in a standardized way (32).
Laparoscopic transhiatal esophagectomy, a revisitation of the procedure popularized in the 70s’ by Orringer and Sloan (33), was first described by DePaula et al. in 1995 (34). Later, a transhiatal esophagectomy through transcervical video-assisted mediastinoscopy combined with laparoscopy was reported (35). A recent systematic review has reported that the laparoscopic transhiatal esophagectomy is associated with a lower median blood loss and a shorter hospital stay compared to the open approach (36). However, this operation has become less popular over the past decade and has been largely replaced by the trans-thoracic approach which also provides an oncological advantage in patients with adenocarcinoma (37).
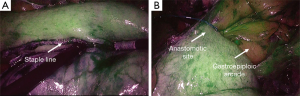
The efficacy of minimally invasive esophagectomy has been demonstrated even in patients treated with neoadjuvant chemoradiotherapy (15,38); however, in patients with advanced bulky tumors before treatment and in those who are candidates to salvage surgery the indication to minimally invasive surgery should be prudent. Radiation damage to the gastric fundus can increase the risk of anastomotic leakage in these patients (39). Microperfusion assessment with indocyanine green fluorescence angiography (Figure 15) may help to establish the best site for the anastomosis (40,41).
From the oncological standpoint, the question whether the minimally invasive techniques represent a viable alternative to the open procedures remains unanswered due to the heterogeneity of the procedures and the lack of long-term data. One meta-analysis found a that the statistically significant increase in lymph node yield associated with minimally invasive esophagectomy did not translate into a survival benefit as no difference was found in 1-, 2-, 3- and 5-year survival rates (42). In a prospective phase II multicenter trial including 104 patients in 17 centers, the 3-year overall survival and recurrence rate were 68% and 33.8%, respectively, following both 3-stage and 2-stage total minimally invasive esophagectomy (43). The most recent 3-year results of the TIME trial showed that the disease-free survival was 35.9% in the open versus 40.2% in the minimally invasive group (44).
Conclusions
Over the past three decades, a marked decrease in postoperative mortality due to better patient selection, improved perioperative care, and concentration of surgical procedures in high-volume centers has been observed. Transthoracic esophagectomy has emerged as the best surgical approach in fit patients. Minimally invasive esophagectomy has added value to this incremental progress by reducing postoperative pain and decreasing the incidence of pulmonary complications.
Both the 3-stage and the 2-stage esophagectomy techniques, either hybrid or total minimally invasive, have proven safe and effective in expert centers and have led to a paradigm shift. Compared to the open surgical approach, the results of minimally invasive esophagectomy appear equivalent in terms of postoperative morbidity and mortality, node retrieval, completeness of resection, and early oncological results. Until further proof of effectiveness and generalizability is reached, the choice of the minimally invasive technique of esophagectomy depends on tumor location, histology, and surgeon’s experience and preference. The modern esophageal surgeon should continue to focus on performing a minimally invasive and maximally effective esophagectomy with low morbidity and mortality rates.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomized controlled trial. Lancet Oncol 2015;16:1090-98. [Crossref] [PubMed]
- Basta YL, Bolle S, Fockens P, et al. The value of multidisciplinary team meetings for patients with gastrointestinal malignancies: a systematic review. Ann Surg Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Bonavina L, Incarbone R, Lattuada E, et al. Preoperative laparoscopy in management of patients with carcinoma of the esophagus and of the esophagogastric junction. J Surg Oncol 1997;65:171-4. [Crossref] [PubMed]
- Bonavina L, Scolari F, Aiolfi A, et al. Early outcome of thoracoscopic and hybrid esophagectomy: propensity-matched comparative analysis. Surgery 2016;159:1073-81. [Crossref] [PubMed]
- Okamura A, Watanabe M, Mine S, et al. Factors influencing difficulty of the thoracic procedure in minimally invasive esophagectomy. Surg Endosc 2016;30:4279-85. [Crossref] [PubMed]
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- Takeuchi H, Kawakubo H, Kitagawa Y. Current status of minimally invasive esophagectomy for patients with esophageal cancer. Gen Thorac Cardiovasc Surg 2013;61:513-21. [Crossref] [PubMed]
- Palanivelu C, Prakash A, Senthilkumar R, et al. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position. Experience of 130 patients. J Am Coll Surg 2006;203:7-16. [Crossref] [PubMed]
- Fabian T, Martin J, Katigbak M, et al. Thoracoscopic esophageal mobilization during minimally invasive esophagectomy: a head-to-head comparison of prone versus decubitus positions. Surg Endosc 2008;22:2485-91. [Crossref] [PubMed]
- Barbera C, Milito P, Punturieri M, et al. Serratus anterior plane block for hybrid transthoracic esophagectomy: a pilot study. J Pain Res 2017;10:73-7. [Crossref] [PubMed]
- Haverkamp L, Seesing MF, Ruurda JP, et al. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus 2017;30:1-7. [PubMed]
- Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 2010;24:1621-9. [Crossref] [PubMed]
- Sgourakis G, Gockel I, Radtke A, et al. Minimally invasive versus open esophagectomy: metaanalysis of outcomes. Dig Dis Sci 2010;55:3031-40. [Crossref] [PubMed]
- Mamidanna R, Bottle A, Aylin P, et al. Short term outcomes following open versus minimally invasive esophagectomy for cancer in England: a population based national study. Ann Surg 2012;255:197-203. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Parameswaran R, Blazeby JM, Hughes R, et al. Health-related quality of life after minimally invasive oesophagectomy. Br J Surg 2010;97:525-31. [Crossref] [PubMed]
- Maas KW, Cuesta MA, van Berge Henegouwen MI, et al. Quality of Life and Late Complications After Minimally Invasive Compared to Open Esophagectomy: Results of a Randomized Trial. World J Surg 2015;39:1986-93. [Crossref] [PubMed]
- Jagot P, Sauvanet A, Berthoux L, et al. Laparoscopic mobilization of the stomach for oesophageal replacement. Br J Surg 1996;83:540-2. [Crossref] [PubMed]
- Bonavina L, Bona D, Binyom PR, et al. A laparoscopy-assisted surgical approach to esophageal carcinoma. J Surg Res 2004;117:52-7. [Crossref] [PubMed]
- Bonavina L. Selected commentary to “Fifty-five minimally invasive esophagectomies: a single-center experience”. Eur Surg 2009;41:194-8. [Crossref]
- Messager M, Pasquer A, Duhamel A, et al. Laparoscopic gastric mobilization reduces postoperative mortality after esophageal cancer surgery: a French nationwide study. Ann Surg 2015;262:817-22; discussion 822-3. [Crossref] [PubMed]
- Briez N, Piessen G, Torres F, et al. Effects of hybrid minimally invasive oesophagectomy on major postoperative pulmonary complications. Br J Surg 2012;99:1547-53. [Crossref] [PubMed]
- Briez N, Piessen G, Bonnetain F, et al. Open versus laparoscopically-assisted oesophagectomy for cancer: a multicentre randomised controlled phase III trial – the MIRO trial. BMC Cancer 2011;11:310. [Crossref] [PubMed]
- Tapias LF, Morse CR. Minimally invasive Ivor Lewis esophagectomy: description of a learning curve. J Am Coll Surg 2014;218:1130-40. [Crossref] [PubMed]
- Maas KW, Biere SS, Scheepers JJ, et al. Minimally invasive intrathoracic anastomosis after Ivor Lewis esophagectomy for cancer: a review of transoral or transthoracic use of staplers. Surg Endosc 2012;26:1795-802. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Nieuwenhuijzen G, et al. Techniques and short-term outcomes for total minimally invasive Ivor Lewis esophageal resection in distal esophageal and gastroesophageal cancers: pooled data from six European centers. Surg Endosc 2017;31:119-26. [Crossref] [PubMed]
- Bonavina L, Laface L, Abate E, et al. Comparison of ventilation and cardiovascular parameters between prone thoracoscopic and Ivor Lewis esophagectomy. Updates Surg 2012;64:81-5. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy. Review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Kusano C, Baba M, Takao S, et al. Oxygen delivery as a factor in the development of fatal postoperative complications after oesophagectomy. Br J Surg 1997;84:252-7. [Crossref] [PubMed]
- Cheng YJ, Chan KC, Chien CT, et al. Oxidative stress during 1-lung ventilation. J Thorac Cardiovasc Surg 2006;132:513-8. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer, a randomized controlled trial (ROBOT trial). Trials 2012;13:230. [Crossref] [PubMed]
- Walther B, Johansson J, Johnsson F, et al. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction. A prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg 2003;238:803-12. [Crossref] [PubMed]
- Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg 1978;76:643-54. [PubMed]
- DePaula AL, Hashiba K, Ferreira E, et al. Laparoscopic transhiatal esophagectomy with esophagogastroplasty. Surg Laparosc Endosc 1995;5:1-5. [PubMed]
- Bonavina L, Incarbone R, Bona D, et al. Esophagectomy via laparoscopy and transmediastinal endodissection. J Laparoendosc Adv Surg Tech A 2004;14:13-6. [Crossref] [PubMed]
- Parry K, Ruurda JP, van der Sluis PC, et al. Current status of laparoscopic transhiatal esophagectomy for esophageal cancer patients: a systematic review of the literature. Dis Esophagus 2017;30:1-7. [PubMed]
- Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000. [Crossref] [PubMed]
- Warner S, Chang YH, Paripati H, et al. Outcomes of minimally invasive esophagectomy in esophageal cancer after neoadjuvant chemoradiotherapy. Ann Thorac Surg 2014;97:439-45. [Crossref] [PubMed]
- Goense L, van Rossum PS, Ruurda JP, et al. Radiation to the gastric fundus increases the risk of anastomotic leakage after esophagectomy. Ann Thorac Surg 2016;102:1798-804. [Crossref] [PubMed]
- Zehetner J, DeMeester SR, Alicuben ET, et al. Intraoperative assessment of perfusion of the gastric graft and correlation with anastomotic leaks after esophagectomy. Ann Surg 2015;262:74-8. [Crossref] [PubMed]
- Yukaya T, Saeki H, Kasagi Y, et al. Indocyanine green fluorescence angiography for quantitative evaluation of gastric tube perfusion in patients undergoing esophagectomy. J Am Coll Surg 2015;221:e37-42. [Crossref] [PubMed]
- Dantoc M, Cox MR, Eslick GD. Evidence to support the use of minimally invasive esophagectomy for esophageal cancer: a meta-analysis. Arch Surg 2012;147:768-76. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Franchetti Y, et al. Minimally invasive esophagectomy. Results of a prospective phase II multicenter trial-The Eastern cooperative oncology group (E2202) study. Ann Surg 2015;261:702-7. [Crossref] [PubMed]
- Straatman J, van der Vielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]