The value of cell block based on fine needle aspiration for lung cancer diagnosis
Introduction
Lung cancer is currently the leading cause of cancer-related morality worldwide (1). Obtaining an accurate diagnosis as soon as possible is very important for the lung cancer patients. Diagnosis of lung cancer can be made in two ways: histopathological diagnosis and cytopathological diagnosis.
Traditional cytopathology is used for differential diagnoses of benign and malignant lesions worldwide. However, accurately diagnosing lung cancer subtype based only on cell morphology can be difficult due to the anaplasia of lung cancer cells. Prior to the 2004 World Health Organization (WHO) classification for lung cancer, no therapeutic implications were associated with distinguishing among histological subtypes of non-small-cell lung carcinomas (NSCLCs) (2). However, due to major therapeutic advances in the lung cancer field, pathological diagnosis has increasing implications for clinicians, particularly with respect to the differential diagnosis of squamous cell carcinoma (SCC) and adenocarcinoma (AC). Numerous drugs focus on specific molecular targets that are primarily associated with either SCC or AC.
Core biopsy and fine needle aspiration (FNA) are the two methods of choice for diagnosing of lung cancer (3). Core biopsies yield histological specimens that can be used for immunohistochemistry (IHC), polymerase chain reaction (PCR) and fluorescence in situ hybridization (FISH), which are useful for precision medicine. FNA has a lower rate of complications than core needle biopsy, but making diagnoses using cytological samples obtained with FNA is more difficult (4-7). For FNA cytological samples, immunocytochemistry (ICC), which should be executed after using the cell block (CB) approach, plays an important role in the differential diagnosis of lung cancer subtypes. At present, according to the 2015 WHO classification of lung cancer, ICC is recommended for all NSCLC cases that cannot be classified as SCC or AC based on morphology alone (2). Similarly, differentiating metastatic tumors of the lung from primary tumors of the lung based only on smears is difficult, and ICC is also needed to resolve these differences.
Here, we studied the utility of CB and ICC based on FNA samples for diagnosing lung cancer.
Methods
Case collection
When CT scan reports show that the patient might have a tumor, clinicians conducted CT-guided percutaneous lung FNA. We collected 526 cytological samples from May to October of 2015 involved 362 males and 164 females, which corresponded to agender ratio of 2.21:1.00. The patient ages ranged widely from 13–92 years, with a median age of 63 years.
Each cytological sample obtained by CT-guided percutaneous lung FNA (22G) was placed into a 50-cc centrifuge tube with a ThinPrep (HOLOGIC Gen-Probe, San Diego, CA, USA) cytology test (TCT) preservation solution after the creation of conventional smears. TCT smears were obtained using the standard process. Subsequently, samples from all 526 cases were subjected to CB, and ICC was performed. The “cytology smear” group includes a TCT smear and a conventional smear from the same patient. If the TCT smear and conventional smear was positive, a positive result was obtained. The diagnosis of the “cytology smear” group was based on the TCT smear and the conventional smear.
The study was approved by the ethics committee of Shanghai Pulmonary Hospital (No. K17-125). Written informed consent was obtained from all patients.
Diagnosis criteria
Smears with malignant cells were regarded as positive. Highly suspicious tumor cells were used to identify samples as suspicious of carcinoma. Smears with no tumor cells or only a small quantity of cells with nuclear atypia were regarded as negative. The classification of positive samples was conducted in accordance with the 2015 WHO classification criteria for lung cancer. All of the cases were diagnosed by two experienced cytopathologists, and another cytopathologist was invited to provide a diagnosis in cases of disagreement between the two primary cytopathologists.
CB and ICC
If there were difficulties making an accurate diagnosis, CBs were generated with the cytological samples obtained by CT-guided percutaneous lung FNA. On the second day, H&E-stained slides were generated, and ICC markers were evaluated. On the third day, the ICC slides were ready for assessment, and a diagnosis was made according to the ICC results. All of the slides were stained with an autostainer using an envision detection system (Leica Biosystems Melbourne Pty Ltd., Melbourne, Australia). Antibodies to the following molecules were used: P40 (P40, 1:500, OriGene), TTF-1 (8G7G3/1, 1:200, Dako), SYN (DAK-SYNAP, 1:100, Dako), CD56 {123C3[5], 1:50, Dako}, CgA (DAK-A3, 1:100, Dako), LCA (2B11 + PD7/26, 1:100, Dako), CK5/6 (D5/16B4, 1:100, Dako) and Ki-67 (MIB-1, 1:100, Dako). The negative and positive controls included in the study were napsin A (IP64, 1:100, OriGene), CDX-2 (DAK-CDX2, 1:100, Dako), villin (ID2C3, 1:1, Dako), CK7 (DV-TL, 1:100, Dako), CK20 (KS20, 1:50, Dako), ER (EP1, 1:40, Dako), PR (P9R636, 1:50, Dako), c-erBb-2 (EP3, 1:200, OriGene), and AFP (polyclonal antibodies, 1:800, Dako). ALK IHC was performed on a VENTANA Medical System. The primary antibody (clone D5F3, VMSI) was incubated on CB sections for 20 minutes. The Optiview DAB IHC Detection Kit (VMSI) and the Optiview Amplification Kit (VMSI) were used according to the manufacturer’s protocol.
Statistical analysis
Statistical analysis, which was conducted using SPSS software (version 17.0, SPSS, Inc., Chicago, IL, USA), included χ2 tests. P values <0.05 were regarded as statistically significant.
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were used to quantify the ICC marker when diagnosing SCC and AC.
Results
No difference in the CB success rate was found between various specimens
Among the 526 cases, 417 cases were positive, 32 cases were suspicious of carcinoma, and 77 cases were negative. In 59 cases, no cellular component was found after CB, the success rate for CB was 88.78%. The positive specimens were diagnosed as SCC, AC, SCLC, NSCLC, not otherwise specified (NSCLC-NOS), poorly differentiated carcinoma, or other malignant tumors. The number and CB success rates for each type of specimen are summarized in Table 1. No statistically significant differences with respect to the CB success rate were found among the positive specimens, the suspicious of carcinoma specimens, and the negative specimens.
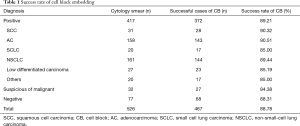
Full table
CB has advantages for distinguishing between benign and malignant tumors
After the CBs were assessed, the rate of positive diagnosis increased from 79.28% to 84.41% (P<0.05); in contrast, the rate of suspected carcinoma decreased from 6.08% to 1.90% (P<0.05; Table 2). We also identified five false-negative cases after performing CB and ICC.
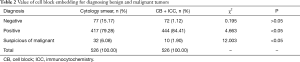
Full table
CB has advantages for determining NSCLC subtypes and identifying metastatic carcinoma of the lung
We determined final subtype diagnoses based on CB and ICC and compared these results with diagnoses based on smears alone (Table 3). The use of CB and ICC decreased the NSCLC diagnoses from 30.61% to 6.27% of the cases (P<0.05). Additionally, in six cases, we obtained conclusions that were different from the initial diagnoses: two cases were diagnosed as AC before CB but subsequently diagnosed as SCC, and four AC cases were misdiagnosed as SCC or SCLC based on smears. We could not distinguish metastatic lung tumors from primary lung tumors based only on smears. After CB and ICC were performed, eight metastatic lung tumors were categorized as four metastatic bowel tumors, three metastatic breast tumors and one metastatic liver tumor (Table 3). We diagnosed metastatic lung tumors not only based on the morphology but also according to the ICC results. Metastatic bowel tumors were positive for CDX-2, villin, and CK20. Metastatic breast tumors were positive for ER, PR, and c-erBb-2, and the metastatic liver tumor was positive for AFP. The ICC for TTF-1 and Napsin A were negative in all of these metastatic tumors.

Full table
TTF-1, Napsin A, P40, and CK5/6 constitute reliable panel for subtyping NSCLC in CB specimens.
The positive rate, sensitivity, specificity, PPV, and NPV for each of these markers are presented in Table 4. TTF-1 is the most specific marker for diagnosing AC and has the best PPV index (specificity: 100%, PPV: 100%). P40 is the most specific marker for diagnosing SCC (specificity: 99.60%) but is occasionally expressed in AC.
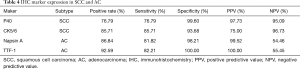
Full table
ALK fusion can be identified in CB specimens
Six (3.73%) of the 161 NSCLC patients exhibited the ALK fusion based on the CB specimens. Using ICC to identify ALK fusions is convenient and inexpensive.
Discussion
FNA and core needle biopsy are two methods used to collect pulmonary lesion specimens. However, the latter method has a higher complication rate for percutaneous transthoracic CT-guided biopsies, such as hemothorax, pneumothorax and hemoptysis (5-7). CT-guided percutaneous lung FNA is a minimally invasive procedure to diagnose lung disease that leads to few complications and relatively little damage (8). However, this procedure also has the disadvantages of providing few cellular specimens and not revealing tumor morphology, which interferes with accurate lung cancer subtyping and prognostic assessment (9). Obtaining an accurate diagnosis is extremely important for clinical treatment. In the current era of precise treatment, the lung cancer therapy chosen is determined by the pathological and molecular diagnoses. Thus, facilitating the efficient use of cytological samples to obtain accurate pathological and molecular diagnoses is critical. Cytological samples are no longer utilized only for diagnosis but are now also used for ICC and molecular testing related to potential targeted therapy. In our hospital, we found that the complication rate from FNA was less than 10%. Clinicians recommend that the patient receive a core biopsy instead of FNA only when the lesion is near the pleura. In this situation, the complication rate of a core biopsy is approximately 25% to 30%, in our hospital, which is consistent with a previous report (5-7).
In our study, we found no statistically significant differences in the CB success rate among positive specimens, suspected carcinoma specimens and the negative specimens. The number of cells is the key factor that determines CB success. If sufficient cells are available, any FNA specimen can successfully be utilized for CB.
False-positive and false-negative diagnoses have been reported in respiratory cytopathology, even for specimens evaluated by highly experienced cytologists (10). We found five false-negative cases in this study. Highly differentiated tumors are difficult to distinguish from epithelial cells, and this difficulty can lead to misdiagnoses. Tumor cells can be squeezed into abnormal shapes, which make their diagnosis by cytopathologists challenging. Moreover, tumor cells might not be easily recognized in the presence of large quantities of blood and mucus. In this study, the utilization of CB for diagnosis increased the positive rate of lung carcinoma diagnoses and decreased the rate of suspected carcinoma diagnoses. CB can facilitate observation of the tumor cell structure in resected specimens.
Distinguishing AC from SCC is important due to clinical observations; for instance, relative to patients with AC, patients with SCC are at increased risk for hemorrhage when treated with bevacizumab. Moreover, patients with AC respond better to pemetrexed than patients with SCC. The subtyping of NSCLC based on cytological smears is possible for AC and SCC with typical cytomorphologies. For poorly differentiated tumors, ICC should be used as an adjuvant method to determine the correct diagnosis.
According to Rekhtman et al. (11), approximately 93% of lung cancer cases can be subtyped into AC and SCC using morphologic criteria alone; in other studies, the diagnosis of AC or SCC can be determined in 50–70% of patients based on cytological specimens (12,13). In our opinion, even experienced cytopathologists may misdiagnose based on cytomorphology alone because lung cancer cells are always poorly differentiated. The cytological features of SCC depend on tumor grade (14). SCC may be divided into two types: keratinizing SCC and non-keratinizing SCC. However, in most cases, SCC of the lung is not keratinized. In non-keratinized cases, CB and ICC should be used for differential diagnosis. The morphology of AC cell clusters is highly variable. And reactive type II pneumocytes (RPII) undergo hyperplasia and reactive changes in response to injury resulting from various conditions, such as infections, interstitial lung diseases, organizing pneumonia, pulmonary drug toxicity, and tuberculosis (10,15-17). A lepidic pattern of AC may be observed in small biopsy specimens but is difficult to diagnose in cytological material (18,19). In the aforementioned situations, it is challenging for cytopathologists to arrive at correct diagnoses. The most important step in avoiding misdiagnosis is to correlate morphological findings with CB and ICC results.
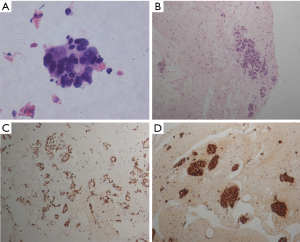
After performing ICC, we found two cases diagnosed as SCC that were actually AC and two cases diagnosed as AC that were SCC. SCCs are known to be positive for CK5/6 and P40 (Figure 1), whereas ACs are positive for TTF-1 and Napsin A (Figure 2) (20). In the 2015 WHO classification recommends the use of ancillary techniques such as ICC to decrease the rate of NSCLC-NOS diagnoses. All these ICC markers that we used for CB specimens are regularly used for surgical specimens. In addition to these markers, we also use P63 for SCC, and SPA and CK7 for AC. In CB specimens, the quantity of cells is not sufficient for the analysis of all ICC markers; therefore, we select the most important markers to enable a diagnosis. In our study, only 33 cases were negative for CK5/6, P40, TTF-1 and Napsin A.
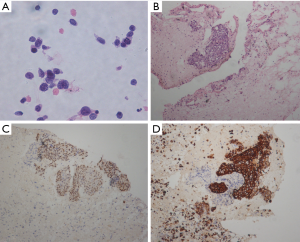
In our study, two other cases of AC were misdiagnosed as SCLC. In these cases, tumor cells were as large as 1.5–3 lymphocytes (12,20). Necrosis was observed in the background, and ghost cells were observed in the smears. A diagnosis can be reliably reached based on cytological morphology, but ICC may be required to confirm this diagnosis. TTF-1, SYN, CD56 and CgA are useful indices (Figure 3). In a small number of SCLC cases, all ICC findings could be negative (20).

Patients with ALK rearrangement comprise 2–5% of all NSCLC cases (21). In our study, we tested all NSCLC cases for ALK rearrangement using IHC. The positive rate was 3.73%, which is consistent with reports in the literature. Screening for ALK rearrangement using IHC (VENTANA) is convenient and economical. Cytological samples can also be screened for ALK rearrangement using CB.
In conclusion, CB and ICC based on CT-guided FNA can provide an accurate diagnosis. Thus, we recommended using CB and ICC to evaluate cytological samples derived from FNA. CB also allows for genetic testing and prognostic assessment via PCR, FISH and IHC (22-25). CB is helpful for diagnosing and subtyping advanced lung cancer, determining precise treatments, and preserving cytological specimens.
Acknowledgements
Funding: This work was supported in part by grants from the Projects of the Shanghai Science and Technology Commission (No. 16ZR1428900).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics committee of Shanghai Pulmonary Hospital (No. K17-125). Written informed consent was obtained from all patients.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Micke P, Mattsson JS, Djureinovic D, et al. The Impact of the Fourth Edition of the WHO Classification of Lung Tumours on Histological Classification of Resected Pulmonary NSCCs. J Thorac Oncol 2016;11:862-72.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Zhang HF, Zeng XT, Xing F, et al. The diagnostic accuracy of CT-guided percutaneous core needle biopsy and fine needle aspiration in pulmonary lesions: a meta-analysis. Clin Radiol 2016;71:e1-10. [Crossref] [PubMed]
- Beslic S, Zukic F, Milisic S. Percutaneous transthoracic CT guided biopsies of lung lesions; fine needle aspiration biopsy versus core biopsy. Radiol Oncol 2012;46:19-22. [Crossref] [PubMed]
- Wang Y, Jiang F, Tan X, et al. CT-guided percutaneous transthoracic needle biopsy for paramediastinal and nonparamediastinal lung lesions: Diagnostic yield and complications in 1484 patients. Medicine (Baltimore) 2016;95:e4460. [Crossref] [PubMed]
- Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138-48. [Crossref] [PubMed]
- Bruno P, Pisani L, Ricci A, et al. Cytology on transbronchial needle aspiration (TBNA): not only for lung cancer. Anticancer Res 2010;30:4769-72. [PubMed]
- Ariza-Prota MA, Bango Álvarez A, Pérez L, et al. From cytology to histology: diagnosis of a relapsed mediastinal lymphoma by endobronchial ultrasound transbronchial histological needle. Respirol Case Rep 2015;3:68-71. [Crossref] [PubMed]
- Beskow CO, Drachenberg CB, Bourquin PM, et al. Diffuse alveolar damage. Morphologic features in bronchoalveolar lavage fluid. Acta Cytol 2000;44:640-6. [Crossref] [PubMed]
- Rekhtman N, Brandt SM, Sigel CS, et al. Suitability of thoracic cytology for new therapeutic paradigms in non-small cell lung carcinoma: high accuracy of tumor subtyping and feasibility of EGFR and KRAS molecular testing. J Thorac Oncol 2011;6:451-8. [Crossref] [PubMed]
- Travis WD. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol 2012;25 Suppl 1:S18-30. [Crossref] [PubMed]
- Loo PS, Thomas SC, Nicolson MC, et al. Subtyping of undifferentiated non-small cell carcinomas in bronchial biopsy specimens. J Thorac Oncol 2010;5:442-7. [Crossref] [PubMed]
- Smith-Purslow MJ, Kini SR, Naylor B. Cells of squamous cell carcinoma in pleural, peritoneal and pericardial fluids. Origin and morphology. Acta Cytol 1989;33:245-53. [PubMed]
- Saad RS, Silverman JF. Respiratory cytology: differential diagnosis and pitfalls. Diagn Cytopathol 2010;38:297-307. [PubMed]
- Grotte D, Stanley MW, Swanson PE, et al. Reactive type II pneumocytes in bronchoalveolar lavage fluid from adult respiratory distress syndrome can be mistaken for cells of adenocarcinoma. Diagn Cytopathol 1990;6:317-22. [Crossref] [PubMed]
- Naryshkin S, Young NA. Respiratory cytology: a review of non-neoplastic mimics of malignancy. Diagn Cytopathol 1993;9:89-97. [Crossref] [PubMed]
- Rudomina DE, Lin O, Moreira AL. Cytologic diagnosis of pulmonary adenocarcinoma with micropapillary pattern: does it correlate with the histologic findings? Diagn Cytopathol 2009;37:333-9. [Crossref] [PubMed]
- Rodriguez EF, Monaco SE, Dacic S. Cytologic subtyping of lung adenocarcinoma by using the proposed International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) adenocarcinoma classification. Cancer Cytopathol 2013;121:629-37. [Crossref] [PubMed]
- Bavikatty NR, Michael CW. Cytologic features of small-cell carcinoma on ThinPrep. Diagn Cytopathol 2003;29:8-12. [Crossref] [PubMed]
- Le T, Gerber DE. ALK alterations and inhibition in lung cancer. Semin Cancer Biol 2017;42:81-8. [Crossref] [PubMed]
- Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol 2015;10:910-23. [Crossref] [PubMed]
- da Cunha Santos G, Saieg MA. Preanalytic parameters in epidermal growth factor receptor mutation testing for non-small cell lung carcinoma: A review of cytologic series. Cancer Cytopathol 2015;123:633-43. [Crossref] [PubMed]
- Neat MJ, Foot NJ, Hicks A, et al. ALK rearrangements in EBUS-derived transbronchial needle aspiration cytology in lung cancer. Cytopathology 2013;24:356-64. [Crossref] [PubMed]
- Zhao C, Li X, Li J, et al. Detecting ALK, ROS1 and RET Fusion Genes in Cell Block Samples. Transl Oncol 2014;7:363-7. [Crossref] [PubMed]


