Optimal cut-off value and clinical usefulness of the Adherence Starts with Knowledge-12 in patients with asthma taking inhaled corticosteroids
Introduction
Asthma is a chronic inflammatory disease of the airways and inhaled corticosteroids (ICS) play a central role for asthma management. However, non-adherence to inhalation therapy is common (1-3). A literature review on adherence to ICS reported that rates of underuse ranged from 24% to 69% (3).
Limiting the benefits of treatment, poor adherence can result in increased frequency of asthma exacerbations, hospitalizations and impaired quality of life (4-6). Therefore, the Global Initiative for Asthma (GINA) stated that follow-up consultations including assessment of patients’ medication adherence and evaluation of inhaler device technique should occur at regular intervals (7). Currently, a variety of methods exist to assess adherence including medication counting (8,9), diaries, electronic monitors (10,11), questionnaires (12-14), and analysis of pharmacy claims databases (15-17). Some simple self-reported adherence questionnaires have been previously studied (12,18,19). However, these questionnaires measure the degree of non-adherence, but they do not address specific barriers that affect adherence (12,18).
The Adherence Starts with Knowledge-20 (ASK-20) survey was developed in the United States with the aims of identifying patient-specific barriers to medication adherence and improving adherence-related communication between medical staff and patients (20). Recently, the ASK-12, a shorter version of the ASK-20, was validated (15). However, the cut-off value of the ASK-12 total score for discriminating medication adherence from non-adherence has yet to be investigated. Establishing a cut-off for the ASK-12 total score will facilitate the identification of patients who are at risk for non-adherence, and who may require clinical assistance in overcoming these barriers. Therefore, this study was conducted to identify the cut-off value for the ASK-12 total score that reflects non-adherence to inhalation therapy and to investigate the utility of the ASK-12 in the clinical setting.
Methods
Subjects
Outpatients with asthma (n=138) were recruited from the asthma and chronic cough clinic of Nagoya City University Hospital, and the Daido Clinic of the Department of Respiratory Medicine, Social Medical Corporation Kojunkai, Daido Hospital. The inclusion criteria were as follows: (I) age ≥16 years; (II) attending regular clinic visits every 1 to 3 months; (III) asthma diagnosed by a respiratory physician according to the 2008 version of GINA guidelines (21); (IV) prescribed inhaled medicines [ICS or ICS and long-acting β2-agonist (LABA) in combination] for at least 6 months; and (V) no change in asthma medication for 3 months. Patients using single maintenance and reliever therapy (SMART) regimens, which enable the use of one inhaler for both maintenance and reliever therapy, were excluded from this study.
Questionnaire
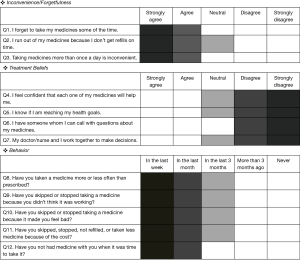
The Japanese version of the ASK-20 has been linguistically validated (22). The ASK-12, a shorter form of the ASK-20 is a 12-item questionnaire consisting of three domains related to medication adherence: inconvenience/forgetfulness, treatment beliefs, and behavior (Figure 1: English version). Responses for each item are scored on a 5-point scale. For items 1–3 and 8–12, higher scores suggest greater adherence difficulties. Items 4–7 are reversely scored so that their final scores are in the same direction as the other 8 items. Scores can range from 12 to 60 with higher scores representing greater barriers to adherence. For each item, the cut-off value indicating a barrier to medication adherence is shown by a dark grey box (Figure 1).
Pharmacy-refill data
An objective measure of refills was expressed as the number of days’ supply during the 6 months reference period (refill frequency × the number of doses per inhaler/number of doses per day based on assumed dose)/number of days in the reference period (13). As defined in previous reports, patients were classified as having good adherence to the inhalation regimen if the refill rate was ≥80%, whereas a rate <80% indicated non-adherence (13,23).
Study protocol
This prospective study was conducted between December 2013 and December 2015. The ASK-12 questionnaire was administered as described elsewhere in the literature (24). Patients were instructed to answer each question regarding inhaled medicines. Pulmonary function tests and fractional exhaled nitric oxide (FeNO: NOA 280i, Sievers Instruments) measurements were performed according to recent recommendations (25), and the Japanese version of Asthma Control Test (ACT) (26) was also implemented on the same day. The cut-off value of the ASK-12 total score used to identify non-adherence to inhalation therapy was determined using pharmacy-refill data as an objective measurement. Using this cut-off value, we were able to investigate patients’ histories of non-adherence with inhalation therapy. Furthermore, to explore the clinical usefulness of the ASK-12 in a longitudinal prospective fashion, asthmatic patients identified as non-adherent (as determined by their ASK-12 cut-off scores) were counseled about how to overcome treatment barriers such as inconvenience and cost of treatment.
The protocol of this study was approved by the Institutional Review Board of Nagoya City University (IRB numbers 1196 and 2121). After receiving an explanation of the study, informed consent was obtained from all patients.
Statistical analysis
Values are expressed as means (± SD), medians (range), or numbers (percentage) as appropriate. All statistical analyses were performed using JUMP 10 Start Statics (SAS Institute Inc., Cary, NC, USA). The effects of switching inhaler prescriptions of 8 patients were analyzed by the Wilcoxon signed rank test. Between-group comparisons were made with Mann-Whitney U-tests, Fisher’s exact probability tests, or chi-square tests for univariate analysis as appropriate. The independent effects of specific factors on non-adherence with inhalation regimens were examined by logistic regression analysis. Variables with a univariate P value <0.15 were selected for inclusion in the logistic regression analysis. For logistic regression analysis, patients were classified as non-elderly (<65 years) and elderly (≥65 years) (27), and the severity of asthma was categorized as mild for 19 patients (step 1 and 2) and moderate-to-severe for 95 patients (step 3, 4 and 5) assessed by GINA treatment step (21). Discriminant analysis was used to determine appropriate cutoff values, using a receiver operating characteristic (ROC) curve. A Spearman rank correlation coefficient was obtained to test the relationships between pharmacy-refill rates and ASK-12 scores. P values <0.05 were considered statistically significant.
Results
Characteristics of respondents
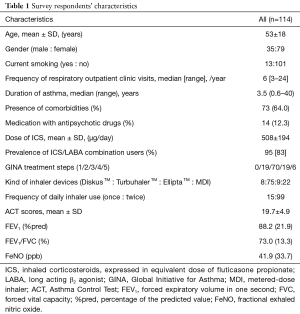
From the 138 potential participants, valid responses were received from 114 (response rate, 82.6%). The respondents were predominantly middle-aged and female. The median disease duration was 3.5 years. All patients received ICS or ICS/LABA combination via DiskusTM, TurbuhalerTM, ElliptaTM or pressurized MDI devices (Table 1). The mean dose of ICS was 508±194 µg.day-1 expressed as the equivalent of fluticasone propionate.
Full table
Relationship between pharmacy-refill rates of inhaled medicine and ASK-12 scores in asthmatic patients
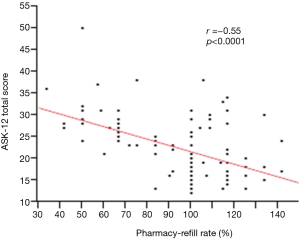
Of the 114 patients, 30 (26.3%) were non-adherent with inhalation regimens as defined by pharmacy-refill rate <80%, and 9 (7.9%) were considered to be over users whose refill rate was over 120%. Relationships between objective measures of pharmacy-refills and ASK-12 scores were evaluated using correlation tests. Refill rates showed a moderate negative correlation within each ASK-12 domain: “Inconvenience/Forgetfulness (Q1–3)” (r=−0.54, P<0.0001), “Treatment Beliefs (Q4–7)” (r=−0.39, P<0.0001), “Behavior (Q8–12)” (r=−0.43, P<0.0001), and ASK-12 total score (r=−0.55, P<0.0001) (Figure 2).
ROC analysis to discriminate non-adherent patients
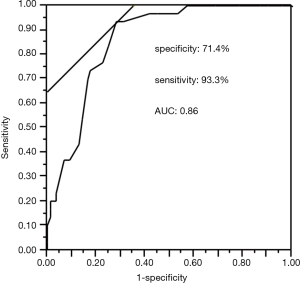
A ROC curve was created to establish the cut-off value of the ASK-12 total score for detecting non-adherence with inhalation treatment as defined by pharmacy-refill rate <0.8. The optimal cut-off value was found to be 23, with 71.4% specificity and 93.3% sensitivity based on the highest sum of specificity and sensitivity achieved for the ASK-12 score. The area under the ROC curve (AUC) was 0.86 (95% CI: 0.79–0.93, P<0.0001) (Figure 3).
Characteristics of adherent versus non-adherent patients
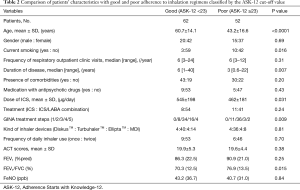
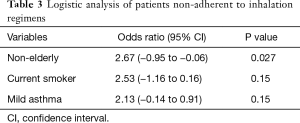
Univariate analysis was conducted to examine differences between patients adherent with their inhalation regimens and those who were not (as classified by the cut-off value of the ASK-12 score (Table 2). Non-adherent asthmatic patients showed a significantly younger age, higher prevalence of current smoking, shorter duration of asthma, less ICS dosing, less severe asthma, and higher FEV1/FVC values than adherent asthmatic patients. No significant differences were found in other clinical indices. Among significant factors, duration of disease, ICS dose, and FEV1/FVC values were excluded from the following multivariate analysis since they were confounding factors for the severity of asthma defined by GINA treatment step. Logistic regression analysis examining age, smoking status, severity of asthma identified younger age as the sole independent predictor of non-adherence to inhalation regimen (odds ratio, 2.67; 95% CI, −0.95 to −0.06; P=0.027) (Table 3). When the patients were classified as adherent or non-adherent according to the pharmacy-refill rate of 80%, the results were almost the same (data not shown).
Full table
Full table
Comparison of ASK-12 items between non-elderly and elderly asthmatic patients
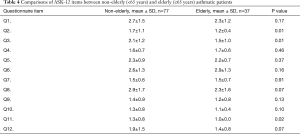
Explanations for younger patients’ non-adherence to inhalation regimens were identified using the ASK-12 (Table 4). The following 3 items in the ASK-12 showed significantly higher scores in nonelderly patients compared to elderly patients: Q2 (1.7 vs. 1.2, P=0.01), Q3 (2.1 vs. 1.5, P=0.01), and Q11 (1.3 vs. 1.0, P=0.02). Furthermore, Q8 and Q12 were marginally higher in nonelderly patients than in elderly patients (2.9 vs. 2.3, P=0.07, 1.9 vs. 1.4, P=0.07, respectively).
Full table
Clinical and practical applications of the ASK-12
Of the 114 patients, eight (7.0%) non-adherent asthmatic patients identified in the study reported barriers in both items Q3 and Q11. As shown in Table 5, switching those patients to less expensive and simpler (once daily) regimens while maintaining an equivalent dose of ICS resulted in significant improvements on both ASK-12 total scores (31.4 to 23.2, P=0.016) and asthma control assessed by ACT (18.4 to 22.1, P=0.046).

Full table
Discussion
To the best of our knowledge, this is the first study to report the cut-off value of the ASK-12 total score needed to identify asthmatic patients who are non-adherent with inhalation regimens. We have also demonstrated for the first time the clinical utility of the ASK-12 in assessing and addressing barriers to inhalation regimen adherence in patients with asthma.
Adherence to medication is critical in the management of asthmatic patients. To assess adherence, it is important to elicit patients’ beliefs and concerns about asthma medications to understand their medication-taking behavior. The ASK-12 was developed to easily identify factors that influence medication adherence in order to improve the efficacy of interventions. In the initial validation study of the ASK-12, Matza et al. (15) observed a weak and marginal negative correlation between the ASK-12 total score and past 6 months pharmacy-refill data, a measure that was used as an objective adherence index (r=−0.20, P=0.059). They studied 112 patients including those with self-reported diagnoses of asthma (n=41), diabetes (n=67) and congestive heart failure (n=1). Compared to this report, our study demonstrated a stronger association between ASK-12 total scores and pharmacy-refill data (r=−0.55, P<0.0001). This may be because we confined our study to objective measures of inhaled medicine use in asthmatic patients. Because the ASK-12 and the ASK-20 ask patients about all of their medicines regardless of their underlying conditions, the ASK-12 is not specifically designed for the assessment of adherence to inhaled medicines. However, all items on the ASK-12 are applicable to inhaled medicines, which distinguish it from the ASK-20 which includes some questions specific to oral medications (20). Given the key role of inhaled medicines and the poorer adherence to ICS than oral treatments in asthma (28), the ASK-12 may be more suitable for the assessment of adherence to inhaled medicine than the ASK-20. We determined the cut-off value of the total score of ASK-12 to be 23. This cut-off score enabled us to identify patients who were non-adherent to inhaled medicine and resulted in a reliable AUC. Fifty-two (46%) of 114 patients with asthma were accordingly classified as non-adherent, which is similar to the rate reported in our previous study (24,29).
Age is associated with adherence to asthma inhalation treatment, with some studies revealing younger patients to be more non-adherent than older adults (29-31). Middle-aged (50 to 64 years old) asthmatic patients were more than 30% less likely to use daily asthma medication compared to adults aged ≥65 years in the United States (32). We have shown that non-adherence to inhalation regimens was more prevalent in non-elderly asthmatic patients than in elderly patients. We analyzed each item score of the ASK-12 with respect to age and demonstrated significantly higher scores on Q2, Q3 and Q11 in nonelderly (<65 years old) patients compared to elderly patients.
To overcome adherence difficulties for eight non-adherent asthmatic patients who reported barriers in both items Q3 and Q11 we switched inhaler prescriptions for these patients to less-expensive and simpler (once-daily) dosing regimens without changing the ICS dose. This resulted in significant improvements in ASK-12 scores and asthma control for these individuals. Frequency of inhaler use may have an effect on medication adherence. A retrospective study of 1,302 asthmatic patients reported that patients taking once-daily ICS dosing were more than 3 times as likely to achieve >75% adherence compared to those taking ICS ≥2 times daily (33). In the present study, however, the frequency of inhaler use was not associated with adherence. This discrepancy is difficult to explain, but may be due to the biased distribution of our participants; 99 (87%) of 114 patients were twice-daily inhaler users. As for cost-related non-adherence, a recent study demonstrated that 1 in 5 families experienced financial burden related to medication cost in the United States (34). A study of 422 African American adult asthmatic women revealed that more than three-fourths of the patients studied were eager to discuss the cost of their asthma medications with their physicians. However, less than half of the patients actually did so (35). The proportion of patients with non-adherence due to medication cost may differ among countries, with Japan having a lower proportion than the United States (36). Although international differences in health insurance systems limit direct comparisons, there is a discrepancy between patients who would like to discuss adherence barriers with their physician and those who actually do it (35). Among our 8 patients, only 1 appealed the burden of medication cost. Our results demonstrate that physicians must give patients opportunities to discuss adherence barriers.
When generalizing the present results, some limitations must be considered. First, the sample size was small. Nevertheless, our study showed that it is possible to quickly identify non-adherent patients and efficiently address barriers to treatment by using the ASK-12. Further studies involving larger numbers of subjects are needed to confirm the clinical usefulness of the ASK-12. Second, we used pharmacy-refill data as our comparator estimate of adherence. This is not a perfect estimator of drug consumption as we cannot verify that drugs dispensed were used at the correct time or dosage. More detail about non-adherence can be obtained by inhalers fitted with electronic chips. However, these devices are bulky, high cost, difficulties related to mechanical malfunctions and training necessary to effectively use that may restrict their use in primary setting. Third, we did not investigate the level of education and that of income in our participants. According to a meta-analysis, age, low income and education were found to predict incomplete adherence in chronically ill patients (37). Finally, because we enrolled subjects who regularly attended clinic visits for more than 6 months, only motivated patients may have been included. Despite the possibility of such selection bias, we still identified non-adherent patients in our study.
In conclusion, we determined the cut-off value of the ASK-12 necessary to identify asthmatic patients who are non-adherent with inhalation regimens. Use of this measure not only enabled us to identify non-adherent patients, but also allowed us to address barriers that contribute to medication non-adherence. The ASK-12 is a practical brief measure with promising clinical utility in assessing and addressing adherence barriers in inhalation regimens.
Acknowledgements
We thank the staff of the outpatient clinic of Daido Hospital for data collection.
Footnote
Conflict of interest: M Takemura, Y Kanemitsu and A Niimi received grants from Novartis Pharma, Astra Zeneca, MSD, and Eisai Co., during the conduct of the study. The authors alone are responsible for the content and writing of the article.
Ethical statement: The protocol of this study was approved by the Institutional Review Board of Nagoya City University (IRB numbers 1196 and 2121). After receiving an explanation of the study, informed consent was obtained from all patients.
References
- Brooks CM, Richards JM, Kohler CL, et al. Assessing adherence to asthma medication and inhaler regimens: a psychometric analysis of adult self-report scales. Med Care 1994;32:298-307. [Crossref] [PubMed]
- Rabe KF, Adachi M, Lai CK, et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol 2004;114:40-7. [Crossref] [PubMed]
- Cochrane MG, Bala MV, Downs KE, et al. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest 2000;117:542-50. [Crossref] [PubMed]
- Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J 2002;19:246-51. [Crossref] [PubMed]
- Hesselink AE, Penninx BW, Wijnhoven HA, et al. Determinants of an incorrect inhalation technique in patients with asthma or COPD. Scand J Prim Health Care 2001;19:255-60. [Crossref] [PubMed]
- Ng TP, Lim TK, Abisheganaden J, et al. Factors associated with acute health care use in a national adult asthma management program. Ann Allergy Asthma Immunol 2006;97:784-93. [Crossref] [PubMed]
- Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J 2015;46:622-39. [Crossref] [PubMed]
- Jónasson G, Carlsen KH, Sodal A, et al. Patient compliance in a clinical trial with inhaled budesonide in children with mild asthma. Eur Respir J 1999;14:150-4. [Crossref] [PubMed]
- Apter AJ, Reisine ST, Affleck G, et al. Adherence with twice-daily dosing of inhaled steroids. Socioeconomic and health-belief differences. Am J Respir Crit Care Med 1998;157:1810-7. [Crossref] [PubMed]
- Bosley CM, Parry DT, Cochrane GM. Patient compliance with inhaled medication: does combining beta-agonists with corticosteroids improve compliance? Eur Respir J 1994;7:504-9. [Crossref] [PubMed]
- Kim C, Feldman HI, Joffe M, et al. Influences of earlier adherence and symptoms on current symptoms: a marginal structural models analysis. J Allergy Clin Immunol 2005;115:810-4. [Crossref] [PubMed]
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67-74. [Crossref] [PubMed]
- Erickson SR, Coombs JH, Kirking DM, et al. Compliance from self-reported versus pharmacy claims data with metered-dose inhalers. Ann Pharmacother 2001;35:997-1003. [Crossref] [PubMed]
- Corsico AG, Cazzoletti L, de Marco R, et al. Factors affecting adherence to asthma treatment in an international cohort of young and middle-aged adults. Respir Med 2007;101:1363-7. [Crossref] [PubMed]
- Matza LS, Park J, Coyne KS, et al. Derivation and validation of the ASK-12 adherence barrier survey. Ann Pharmacother 2009;43:1621-30. [Crossref] [PubMed]
- Steiner JF, Koepsell TD, Fihn SD, et al. A general method of compliance assessment using centralized pharmacy records. Description and validation. Med Care 1988;26:814-23. [Crossref] [PubMed]
- Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care 1999;37:846-57. [Crossref] [PubMed]
- Svarstad BL, Chewning BA, Sleath BL, et al. The Brief Medication Questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ Couns 1999;37:113-24. [Crossref] [PubMed]
- Wetzels G, Nelemans P, van Wijk B, et al. Determinants of poor adherence in hypertensive patients: development and validation of the "Maastricht Utrecht Adherence in Hypertension (MUAH)-questionnaire". Patient Educ Couns 2006;64:151-8. [Crossref] [PubMed]
- Hahn SR, Park J, Skinner EP, et al. Development of the ASK-20 adherence barrier survey. Curr Med Res Opin 2008;24:2127-38. [Crossref] [PubMed]
- Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 2008;31:143-78. [Crossref] [PubMed]
- Atsuta R, To Y, Sakamoto S, et al. Assessing barriers to medication adherence using the Adherence Starts with Knowlegde 20 questionnaire for Japanese adult patients with bronchial asthma receving inhaled corticosteroid-containing medications. Therapeutic Research 2015;36:341-53.
- Vestbo J, Anderson JA, Calverley PM, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax 2009;64:939-43. [Crossref] [PubMed]
- Takemura M, Mitsui K, Ido M, et al. Impact of a network system for providing proper inhalation technique by community pharmacists. J Asthma 2012;49:535-41. [Crossref] [PubMed]
- American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005;171:912-30. [Crossref] [PubMed]
- Hasegawa T, Koya T, Sakagami T, et al. Efficacy of using the Japanese version of the asthma control test for determing the level of asthma control in clinical settings. Allergol Int 2012;61:609-17. [Crossref] [PubMed]
- Inoue H, Niimi A, Takeda T, et al. Pathophysiological characteristics of asthma in the elderly: a comprehensive study. Ann Allergy Asthma Immunol 2014;113:527-33. [Crossref] [PubMed]
- Rand C, Bilderback A, Schiller K, et al. Adherence with montelukast or fluticasone in a long-term clinical trial: results from the mild asthma montelukast versus inhaled corticosteroid trial. J Allergy Clin Immunol 2007;119:916-23. [Crossref] [PubMed]
- Takemura M, Kobayashi M, Kimura K, et al. Repeated instruction on inhalation technique improves adherence to the therapeutic regimen in asthma. J Asthma 2010;47:202-8. [Crossref] [PubMed]
- Bender BG, Pedan A, Varasteh LT. Adherence and persistence with fluticasone propionate/salmeterol combination therapy. J Allergy Clin Immunol 2006;118:899-904. [Crossref] [PubMed]
- Bidwal M, Lor K, Yu J, et al. Evaluation of asthma medication adherence rates and strategies to improve adherence in the underserved population at a Federally Qualified Health Center. Res Social Adm Pharm 2017;13:759-66. [PubMed]
- Deshpande M, Chewning B, Mott D, et al. Asthma medication use among U.S. adults 18 and older. Res Social Adm Pharm 2014;10:e113-23. [Crossref] [PubMed]
- Wells KE, Peterson EL, Ahmedani BK, et al. Real-world effects of once vs greater daily inhaled corticosteroid dosing on medication adherence. Ann Allergy Asthma Immunol 2013;111:216-20. [Crossref] [PubMed]
- Cohen RA, Kirzinger WK. Financial burden of medical care: a family perspective. NCHS Data Brief 2014.1-8. [PubMed]
- Patel MR, Wheeler JR. Physician-patient communication on cost and affordability in asthma care. Who wants to talk about it and who is actually doing it. Ann Am Thorac Soc 2014;11:1538-44. [Crossref] [PubMed]
- Hirth RA, Greer SL, Albert JM, et al. Out-of-pocket spending and medication adherence among dialysis patients in twelve countries. Health Aff (Millwood) 2008;27:89-102. [Crossref] [PubMed]
- DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 2004;42:200-9. [Crossref] [PubMed]


