Adult-onset Langerhans cell histiocytosis of the sternum
Introduction
Langerhans cell histiocytosis (LCH) is a rare disease, predominantly prevalent in children. The incidence of adult-onset LCH is estimated about 1/560,000 adults, and rare occurrences lead to misdiagnosis (1). Herein, we report a case of sternal LCH in a young adult and review nomenclature/classification, clinical manifestations, pathological findings, possible differential diagnosis, and consensus treatment options.
Case presentation
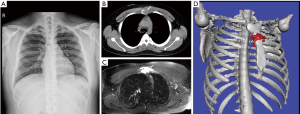
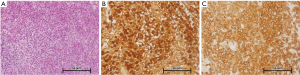
A 20-year-old Asian male presented to our hospital with progressive, spontaneous chest tightness for 3 months, without chest wall contusion. He complained of polydipsia and polyuria for 6 months. Physical examination revealed severe tenderness to touch over the sternum body and close to the manubrium. Blood hemogram revealed leukocytosis (white blood cell count: 13,140 cells/mm3). The urine output was 6,950 mL/day and the specific gravity and osmolarity of urine were 1.002 and 95 mOsm respectively. The C-reactive protein level was 1.49 mg/dL, and erythrocyte sedimentation rate was 34 mm/hour. Chest roentgenogram was negative (Figure 1A). Chest computed tomography revealed a punch-out osteolytic lesion of the sternum (Figure 1B), and magnetic resonance imaging (MRI) of the sternum (Figure 1C) showed a 5 cm × 3.3 cm lobulated soft tissue mass with bony destruction. The surrounding soft tissue involvement was identified by three-dimensional reconstruction and image fusion (Figure 1D). Computed tomography-guided aspiration biopsy was completed. Microscopic examination (Figure 2A) shows loose aggregates of histiocytic appearing cells in a mixed inflammatory background with focal prominent eosinophilia. The Langerhans’ cells have ovoid to reniform nuclei with a longitudinal groove. As for immunohistochemistry, the Langerhans’ cells stained diffuse strong positivity for S-100 (Figure 2B), and CD1a (Figure 2C), and focal patch positive with CD68. These cells fail to express CD45 and cytokeratin. Therefore, the patient was diagnosed with LCH. The brain MRI, whole-body bone scanning, and positron emission tomography were all negative for extra-sternal invasion. He received surgical curettage and adjuvant radiation therapy with good recovery. He is now undergoing regular out-patient department following up at 1st month, 3rd month, 6th month and 1 year after discharging from our hospital and showed no evidence of local recurrence.
Discussion
LCH is a rare disease of abnormally histiocytic proliferation with single (SS-LCH) and/or multiple system (MS-LCH) involvement. Other nomenclature includes histiocytosis X, eosinophilic granuloma, Hand-Schüller-Christian disease (diabetes insipidus, exophthalmos, and lytic bony lesions), Abt-Letterer-Siwe disease, Hashimoto-Pritzker disease, and Congenital self-healing reticulohistiocytosis (2). Most LCH patients are children <4 years old, with a 2:1 ratio of males to females (3). The definition of MS-LCH is the LCH with two or more organs/systems involvement and obtains poorer prognosis and a rapid deterioration than SS-LCH. Poor prognostic factors such as pulmonary LCH, liver function impairment, splenomegaly or bone marrow/hematopoietic abnormity had been reported (1). Patients with MS-LCH with risk organ involvement also obtained higher mortality rate and shorten survival (1,3,4).
Previous study had reported that more than 50% of investigated specimens contained a BRAFV600E gene mutation (4). Although the mechanism and etiology of expansion of these myeloid dendritic cells is still unclear, recent studies have proven that the CD34(+) progenitors in bone marrow contributed to CD1c(+) dendritic cells and CD14(+) monocytes in blood, and give rise to LCH with CD207 (Langerin) and CD1a positivity (5,6).
Clinical manifestations of LCH vary by the affected site(s). Skeletal involvement is the most commonly involved organ, contributing to >57–75% of LCHs. Hence, local bone pain is the most common feature of LCH. Howarth et al. reported that among all the patients with bone lesions included in their study, the most frequent osseous invasion sites were the skull (29.9%), proximal femur (12.4%), and ribs (11.1%) (7). Conversely, only 2/314 cases (0.6%) invaded the sternum; other involved organs included the skin (36.9–39%), lymph nodes (19%), liver (16%), spleen (13%), oral mucosa (13%), lung (10–14%), and central nervous system (6–16%) (7-9). Other symptoms of LCH reported were relatively nonspecific, including dyspnea (14%), malaise, painful scalp lump (9%), spontaneous pneumothorax (7%), and/or diabetes insipidus (6%) (7).
Diagnosis of LCH depends on the patient history, physical examination, imaging studies, histopathology, immunohistochemistry, and electron microscopy. Considering the rarity of SS-LCH of bone and its similarity to radiological osteolytic lesions, it is difficult to differentiate other primary or metastatic bone tumors and benign lesions. Hence, biopsy of these osteolytic lesions is mandatory. Definitive diagnosis in our case was made based on clinical and pathological evidence with at least one of the following findings: Langerin positivity (CD207), CD1a positivity, or presence of Birbeck granules, which have a “tennis racket”-like granular appearance under electron microscopy (4).
To our knowledge, only 13 patients with SS-LCH of the sternum have been reported (10-12). It is interesting that most SS-LCH patients were female (male:female:unknown ratio, 2:9:2), which was in direct contrast to the male to female LCH ratio. Among these 13 patients, only 5 were adults, and all of them were female. Two patients received partial sternotomy, 2 patients only received radiation, and 1 patient received surgical curettage. All these patients recovered well without focal recurrence. Consequently, there is no definitive treatment for SS-LCH.
The current consensus for treating SS-LCH and MS-LCH with bony involvement has been promoted by Girschikofsky et al. and the Histiocytosis Association (4). For SS-LCH with bone lesions, local treatment, such as biopsy, curettage, or intralesional steroid injection, produced more potential benefits than complete excision of the affected bone. Systemic therapy should be considered for MS-LCH, multifocal SS-LCH, or “special-site” SS-LCH, such as vertebral lesions with intraspinal extension or craniofacial bone involvement with soft tissue extension. For SS-LCH without risk of organ involvement, oral methotrexate, azathioprine, or thalidomide is suggested. For MS-LCH treatment, systemic therapy with cytarabine, etoposide, or vinblastin/prednisolone is recommended.
Conclusions
Adult-onset LCH of the sternum is a rare disease that can be easily misdiagnosed. Radiation therapy following curettage is an effective treatment choice for SS-LCH of the skeletal system with surrounding soft tissue invasion. Chest physicians should be alert to abnormal bone pain of the thoracic wall with unexplainable diabetes insipidus.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Pierro J, Vaiselbuh SR. Adult Langerhans cell histiocytosis as a diagnostic pitfall. J Clin Oncol 2016;34:e41-5. [Crossref] [PubMed]
- Histiocytosis syndromes in children. Writing group of the histiocyte society. Lancet 1987;1:208-9. [PubMed]
- Lian C, Lu Y, Shen S. Langerhans cell histiocytosis in adults: a case report and review of the literature. Oncotarget 2016;7:18678-83. [Crossref] [PubMed]
- Girschikofsky M, Arico M, Castillo D, et al. Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis 2013;8:72. [Crossref] [PubMed]
- Milne P, Bigley V, Bacon CM, et al. Hematopoietic origin of Langerhans cell histiocytosis and Erdheim Chester disease in adults. Blood 2017;130:167-75. [Crossref] [PubMed]
- Durham BH, Roos-Weil D, Baillou C, et al. Functional evidence for derivation of systemic histiocytic neoplasms from hematopoietic stem/progenitor cells. Blood 2017;130:176-80. [Crossref] [PubMed]
- Howarth DM, Gilchrist GS, Mullan BP, et al. Langerhans cell histiocytosis: diagnosis, natural history, management, and outcome. Cancer 1999;85:2278-90. [Crossref] [PubMed]
- Héritier S, Emile JF, Barkaoui MA, et al. BRAF mutation correlates with high-Risk Langerhans cell histiocytosis and increased resistance to first-line therapy. J Clin Oncol 2016;34:3023-30. [Crossref] [PubMed]
- Aricò M, Girschikofsky M, Genereau T, et al. Langerhans cell histiocytosis in adults. Report from the International registry of the histiocyte society. Eur J Cancer 2003;39:2341-8. [Crossref] [PubMed]
- Park TH, Kim JK, Oh TY, et al. Solitary Langerhans cell histiocytosis arising from sternum: a case report. J Pediatr Surg 2012;47:e9-12. [Crossref] [PubMed]
- Kontogeorgakos VA, Papachristou DJ, Malizos KN. Eosinophilic granuloma of the sternum in a child treated with closed biopsy. Pediatr Int 2014;56:417-9. [Crossref] [PubMed]
- Zhang Y, Li JZ, Hao YJ, et al. Sternal tumor resection and reconstruction with titanium mesh: a preliminary study. Orthop Surg 2015;7:155-60. [Crossref] [PubMed]


