Tuberculous tracheobronchial stenosis: avoiding resection—when less is more
Introduction
Despite international efforts to eradicate tuberculosis, one third of the world’s population is infected (1). Infection of tracheobronchial tree, termed endobronchial tuberculosis (EBTB), exists in 10–40% of patients with pulmonary involvement. Tracheobronchial stenosis is often a result of EBTB. Incidence of stenosis may reach 68% within 6 months of disease progression and more than 90% long term (2). This process may irreversibly impair lung physiology resulting in respiratory failure and death.
Proposed etiologies of EBTB include implantation from adjacent parenchyma, erosion through infected lymph nodes, and peri-bronchial seeding by hematogenous spread. Histologically, the initial insult is mucosal edema with submucosal lymphocytes and congestion followed by granuloma formation and ulceration. Fibrosis and contractures result in stenosis. Damage to cartilaginous rings eventually leads to mechanical collapse, obstruction, and distal atelectasis (2).
Case presentation
A 30-year-old woman was referred to our surgical clinic with complaints of pain along her left inferior rib cage, cough, and dyspnea. She completed a course of triple-drug antituberculous therapy after sputum cultures grew Mycobacterium tuberculosis, confirming active pulmonary involvement.
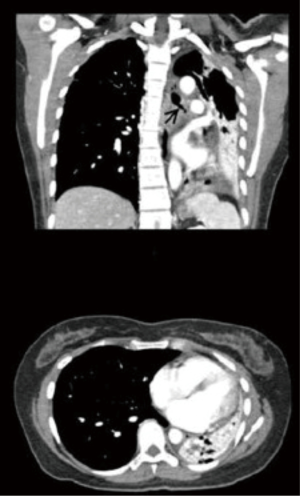
Serial chest radiographs provided by the referring physician showed progressive collapse of the left lower lobe. A computed tomography (CT) scan confirmed this finding along with stenosis of the left main bronchus and left-ward mediastinal shift (Figure 1). Recent bronchoscopy reported tight narrowing at the proximal left main bronchus with inability to pass the scope. Biopsies were negative for malignancy and Mycobacteria. Traditional surgical approaches for this proximal lesion would include pneumonectomy, sleeve lobectomy, or endobronchial stenting. We decided to attempt endoscopic endobronchial treatment to preserve lung parenchyma.
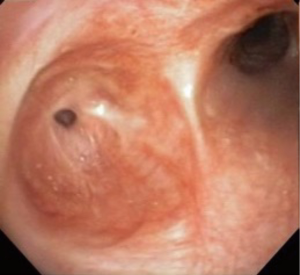
Bronchoscopy confirmed a normal trachea, carina and right bronchial tree. The left main bronchus demonstrated a proximal 95% stenosis with <3 mm opening (Figure 2). Graded hydrostatic balloon dilation and argon laser fulguration was carried out. Upon completion, the bronchus accepted a 14-mm balloon. A stent was not placed to allow healing while avoiding foreign body in this young patient. She was extubated and discharged home.
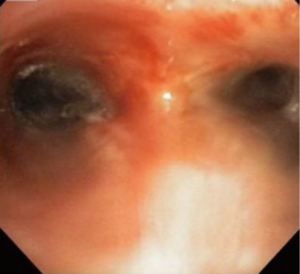
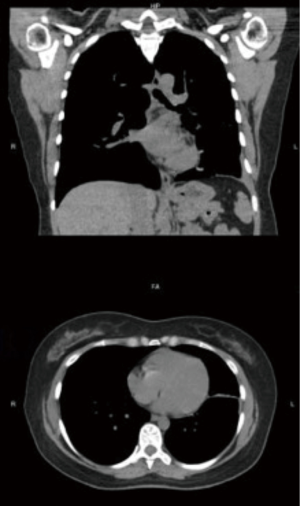
The patient’s symptoms resolved and seven weeks after intervention she underwent surveillance bronchoscopy. Examination revealed healed segment of bronchus and only minimal re-stenosis (Figure 3). The distal airway was patent with good re-expansion of lung parenchyma which was also noted on repeat CT scan (Figure 4). Six months following the initial procedure she again required balloon dilation and argon fulguration of recurrent narrowing. At this time, there was a 2-mm-thick web-like stenosis in the left bronchus. This responded well to repeat therapy with resulting diameter of 15 mm. She was extubated following the procedure and had an uneventful recovery without re-stenosis or need for re-intervention on 3-year bronchoscopic follow-up.
Comment
Once significant tuberculous tracheobronchial stenosis has developed, medical therapy is not sufficient and surgical treatment has been advocated. Many patients have proximal bronchial involvement necessitating a sleeve lobectomy or even pneumonectomy. Those with short segment stenosis and intact lung function may be candidates for sleeve resection. Complications of this procedure include anastomotic leak, restenosis and respiratory failure. The greatest benefit of surgery, when compared with endoscopic therapies, is a lower re-intervention rate (3). Surgery may not be feasible with compromised pulmonary reserve or significant medical comorbidities. Alternative options include stent placement, balloon dilation, and laser fulguration.
Metallic or silicone stents have been shown to be efficacious but not curative. Long-term placement has risk of infection, migration, fracture, and in-stent stenosis. Stent removal has traditionally been performed at 6–18 months for benign disease (4). Over time, removal becomes difficult, or even impossible, due to mucosal hyperplasia and granulation. Additional risks include erosion, perforation, and hemorrhage. Premature removal carries risk of airway collapse requiring emergency re-intervention. More commonly, a gradual re-stenosis requires elective replacement. Stent removal failure rates continue to be as high as 76% while stent re-insertion involves repeated trauma leading to more airway scarring (5).
Successful therapy is a delicate balance between avoiding premature removal while preventing unnecessarily prolonged use.
Balloon dilation, when appropriate, can provide immediate relief. Alone, it carries re-stenosis rates as high as 95% at 1 year (6). Complications include airway laceration, bleeding, pneumothorax, and mediastinitis. Argon laser fulguration is another option. This gas, when ionized by electricity, delivers currents at high temperatures to destroy tissue within the endobronchial tree (7). Low et al. published a series of 21 patients with tuberculous tracheobronchial stenosis who underwent bronchoscopy followed by combination of techniques including balloon dilation, laser, and stenting. The interventions provided immediate symptomatic relief in all patients with repeat procedures necessary in 19% (8). By avoiding direct application of heat, liquid nitrogen cryoablation is a promising new alternative which completely avoids the complications associated with thermal injury. Cryotherapy involves the controlled application of freezing temperatures (−196 °C) directly to target cells. Once the temperature of targeted cells drops below a critical value, intra cellular and extra cellular ice crystals form causing cellular death as the crystals thaw (9). Benefits over thermal modalities include highly selective cellular destruction allowing preservation of the extra cellular matrix to enhance normal regrowth with reduced scarring, reduced injury to cartilage tissue protected by its naturally higher water content further maintaining normal structural integrity and reduced bleeding complications due to vasoconstriction and cryothrombosis induced by low temperatures (9).
The intervention used for tuberculous tracheobronchial stenosis must be tailored in a patient-specific fashion. We present a case of a young woman with no additional medical problems and successful treatment with the “less is more” approach. Surgical resection, which would remove affected tissue, will remain an option but foregoing it early in the course of disease allows for preservation of functional lung parenchyma distal to the lesion. Avoidance of stenting as a first line therapy delays commitment to multiple traumatic re-interventions while still allowing for this and other more aggressive surgical options to be available in the future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The patient has approved written informed consent prior to submission.
References
- Centers for Disease Control and Prevention. Global Tuberculosis. Updated 2017 Feb 16, cited 2015 Oct 4. Available online: http://www.cdc.gov/tb/statistics/
- Kashyap S, Solanki A. Challenges in endobronchial tuberculosis: from diagnosis to management. Pulm Med 2014;2014:594806.
- Watanabe Y, Murakami S, Oda M, et al. Treatment of bronchial stricture due to endobronchial tuberculosis. World J Surg 1997;21:480-7. [Crossref] [PubMed]
- Verma A, Um SW, Koh WJ, et al. Long-term tolerance of airway silicone stent in patients with post-tuberculosis tracheobronchial stenosis. ASAIO J 2012;58:530-4. [Crossref] [PubMed]
- Eom JS, Kim H, Park HY, et al. Timing of silicone stent removal in patients with post-tuberculosis bronchial stenosis. Ann Thorac Med 2013;8:218-23. [Crossref] [PubMed]
- Lee KH, Ko GY, Song HY, et al. Benign tracheobronchial stenoses: long-term clinical experience with balloon dilation. J Vasc Interv Radiol 2002;13:909-14. [Crossref] [PubMed]
- Erelel M, Yakar F, Yakar A. Endobronchial tuberculosis with lobar obstruction successfully treated by argon plasma coagulation. South Med J 2009;102:1078-81. [Crossref] [PubMed]
- Low SY, Hsu A, Eng P. Interventional bronchoscopy for tuberculous tracheobronchial stenosis. Eur Respir J 2004;24:345-7. [Crossref] [PubMed]
- Ma Q, Shi B, Tian Y, et al. Fibrobronchoscopic cryosurgery for secondary malignant tumors of the trachea and main bronchi. Thorac Cancer 2016;7:459-66. [Crossref] [PubMed]


