Hemangiopericytoma 11 years later: delayed recurrence of a rare soft tissue sarcoma
Introduction
Hemangiopericytoma (HPC) is a rare disease entity of soft tissue sarcomas that originates in the pericytes in the walls of capillaries, and was first described in 1942 by Arthur Purdy Stout and Margaret R. Murray. They have a high propensity for recurrence (1). They have since been classified as a fibroblastic neoplasm, similar to solitary fibrous tumours. Median age of presentation is in the 40s (2), although occurrence can vary between 20–70 years of age. Virtually any part of the body may be involved but typically over the lower extremities, lung, pleura, and meninges.
Case presentation
A 41-year-old female was referred to our Cardiothoracic Surgery Unit for an intrathoracic tumour measuring 4 cm × 3 cm on the inner aspect of her left posterior 3rd rib. She had two previous intracranial resections for meningeal HPC in 2004 and 2008, as well as adjuvant radiotherapy in 2008. She was well until June 2015 when she started experiencing pain in her left hip. A positron emission tomography/computed tomography (PET/CT) scan was done, showing fluorodeoxyglucose (FDG)-avid lesions at her left femur and left 3rd rib. In September of 2015, she underwent a resection of the proximal portion of her left femur and reconstruction of the proximal femur with a prosthesis implant at a private center. The mass was histologically proven to be HPC. She was subsequently referred to our unit for resection of the left 3rd rib tumour (Figures 1,2).
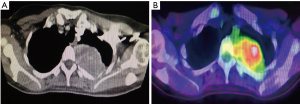
We attempted a video-assisted thoracoscopic surgery (VATS) resection of her tumour in October of 2015. On thoracoscopic examination, we concluded that a VATS resection would be complex, due to the size of the tumour and the abutment to the thoracic vertebra. Therefore, posterolateral thoracotomy was performed and the tumour excised en bloc along with left posterior 3rd rib and surrounding musculature. It was successfully resected off the corresponding vertebra where a clear margin between the vertebra and tumour was seen (Figure 3).
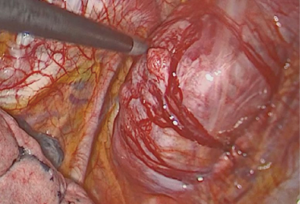
She was discharged home on the 4th post-operative day with minimal pain. There were no neurosensory or motor deficits. She was subsequently followed up in our clinic 2 weeks after her discharge, and was well.
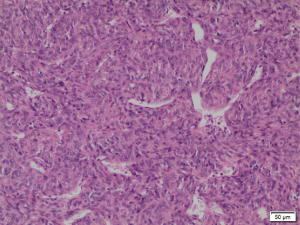
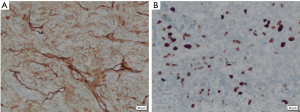
Histopathological studies of the resected segment showed malignant epithelioid-shaped cells in solid sheets, with thin-walled branching vessels between the cells (Figure 4). Mitotic figures were easily detected. The fibrous capsule showed no invasion, and all the resected margins were clear of tumour. Immunohistochemistry staining was positive for CD31, CD34 (Figure 5A) and Bcl-2; while staining for S100 and actin were negative. Ki67 proliferation index was raised as high as 40–50% (Figure 5B), in keeping with a recurrent HPC which has been fully resected.
She was subsequently referred to the Oncology Department for further treatment and was offered chemotherapy. She was however informed of the limited response of adjuvant chemotherapy and decided to decline chemotherapy.
Discussion
A search of the literature revealed a paucity of case reports. One large study published in 1998 from the University of Texas describes only 36 patients over a 20-year period (2). Another, published in 2002 from the Memorial Sloan-Kettering (MSK) Cancer Center, described 25 patients over a 16-year period, where only 3 (12%) were metastatic (3). The Mayo Clinic published a series in 1998 of 34 patients with HPC over the course of 20 years. Surgery was the treatment modality of choice and recurrence rates were reported at 29% (1) (Table 1).
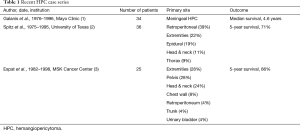
Full table
The diagnosis of HPC has been difficult in the past, prior to the usage of routine immunohistochemistry. On histopathological examination, HPC have a typical sponge-like sinusoidal vasculature and staghorn-shaped blood vessels, that are bounded by spindle-shaped cells. The cells are largely undifferentiated, and contain arrays of intermediate filaments. Presence of the basement membrane substance is necessary on ultrastructural analysis. They are negative for keratins and epithelial membrane antigen (EMA) (3). Electron microscopy and genetic studies have been suggested to further aid in the accurate diagnosis of this tumour. As HPCs are rare tumours, there have been little guidelines on the best way to approach these tumours. Because even benign appearing solitary tumours can be locally recurrent and metastatic, therefore wide resection of both benign and malignant tumours are recommended. Preoperative vascular studies and arterial embolization should be considered because of the known bleeding risk with resection (4,5).
No evidence suggests that adjuvant chemotherapy is beneficial. If the HPC appears malignant histologically, adjuvant radiation therapy may be considered (6,7). Adjuvant therapy for this patient is limited as chemotherapy is ineffective and radiotherapy is not suitable for the thoracic cavity. Patients with a primary tumor who undergo complete resection, a 5-year survival rate is 89–100%. For patients with HPCs of an extremity, the local recurrence rate is 0–6%, and the distant metastasis rate is 0–19% (3,8,9).
Complete surgical resection with clear margins remain the best treatment for this patient and offer best chance of survival. Her subsequent CT scan did not reveal recurrence at the resection site. However, given the history of multiple recurrence at different sites from the primary tumor, the long-term outcome of this patient is less favourable due to high possibility of future recurrences.
This patient had extracranial recurrence 11 years after complete resection of the primary tumour. Therefore, any patient with a history of treated HPC and develops new symptoms should be worked up for recurrence. This is regardless of the length of disease-free interval, as our case study suggested. Patients should be informed of the risk of recurrence, and to seek medical consultation should they develop symptoms such as headaches or vague pains. There has yet to be a standardized follow-up regime due to the rarity of these tumours.
Conclusions
HPC remains a rare soft tissue sarcoma with high recurrence rate. Planned VATS evaluation and resection is possible provided complete resection with clear surgical margins can be achieved, as clear surgical margins offer the best chance of survival.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Content: Informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Galanis E, Buckner JC, Scheithauer BW, et al. Management of recurrent meningeal hemangiopericytoma. Cancer 1998;82:1915-20. [Crossref] [PubMed]
- Spitz FR, Bouvet M, Pisters PW, et al. Hemangiopericytoma: a 20-year single-institution experience. Ann Surg Oncol 1998;5:350-5. [Crossref] [PubMed]
- Espat NJ, Lewis JJ, Leung D, et al. Conventional hemangiopericytoma: modern analysis of outcome. Cancer 2002;95:1746-51. [Crossref] [PubMed]
- Penel N, Amela EY, Decanter G, et al. Solitary fibrous tumors and so-called hemangiopericytoma. Sarcoma 2012;2012:690251.
- Lorigan JG, David CL, Evans HL, et al. AJR Am J Roentgenol 1989;153:345-9. [Crossref] [PubMed]
- Lee SJ, Kim ST, Park SH, et al. Successful use of pazopanib for treatment of refractory metastatic hemangiopericytoma. Clin Sarcoma Res 2014;4:13. [Crossref] [PubMed]
- Delgado M, Pérez-Ruiz E, Alcalde J, et al. Anti-angiogenic treatment (sunitinib) for disseminated malignant haemangiopericytoma: a case study and review of the literature. Case Rep Oncol 2011;4:55-9. [Crossref] [PubMed]
- Demicco EG, Park MS, Araujo DM, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol 2012;25:1298-306. [Crossref] [PubMed]
- Akisue T, Matsumoto K, Kizaki T, et al. Solitary fibrous tumor in the extremity: case report and review of the literature. Clin Orthop Relat Res 2003.236-44. [Crossref] [PubMed]


