Five reasons for caution in advocating low-dose computerized tomographic lung cancer screening
Failure to reproduce the benefit of National Lung Cancer Screening Trial (NLST)
The NLST choice of controls was based on a sound rationale: “Chest radiography was chosen as the screening method for the control group because radiographic screening was being compared with community care (care that a participant usually receives) in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. The designers reasoned that if the PLCO trial were to show a reduction in lung-cancer mortality with radiographic screening, a trial of low dose CT screening in which a community-care group was the control would be of less value, since the standard of care would have become screening with chest radiography. Nevertheless, the choice of radiography precludes a direct comparison of low-dose CT with community care. Analysis of the subgroup of PLCO participants who met the NLST criteria for age and smoking history indicated that radiography, as compared with community care, does not reduce mortality” (1).
A 30K subgroup of persons in the PLCO trial who met the age and smoking screening criteria of the NLST were evaluated to compare the effect of null to chest radiograph (CR)-screening (2). Their report of an unprecedented differential lung cancer incidence constitutes grounds for questioning their equivalence: the number of cases of lung cancer identified in the null (unscreened) group [520] exceeded that in the CR-screened group [518]. Previous experience in large CR screening trials demonstrated that 22–24% more cases were identified in the CR-screened vs. null controls (3). Furthermore, as expected in a large population, while the annual reported intergroup difference in lung cancer fatality was trivial in most years, two annual reports cited fatality differences of 39 and 44 cases (3). Individually and in combination, these outlier data are difficult to credit. They strongly suggest erratic reportage. Mortality in the CR-screened and unscreened groups did not materially differ.
None of three reporting European trials, each of which employed null controls, reproduced the benefit reported in the NLST (4,5). They were collectively one-fifth the size of the NLST—10.7K vs. 53.5K subjects. They reported overall greater morbidity, more operations for benign conditions, and higher mortality in the low-dose computerized tomographic (LDCT) cohorts.
Surgical morbidity/mortality
Centers of excellence, screening selected candidates, achieved markedly favorable results. Surgical mortality for pulmonary resection in the NLST was 1% (1). The International ELCAP reported an operative mortality of 0.6% (6). To what extent these figures will be reproduced under less favorable circumstances is not known. The National Cancer Database reported on 119K persons with surgically managed non-small cell lung cancer (NSCLC): 30-day mortality was 3.4% overall; 2.6% for lobectomy; 4.2% for wedge resection; 4% for extended lobectomy/bilobectomy and 8.5% for pneumonectomy. Of patients who underwent lobectomy, 9.1% had an extended length of stay (7).
Overdiagnosis
By definition, lung cancer survival in overdiagnosed persons is 100%. Veronesi et al. advanced the concept of employing tumor volume doubling time (TVDT) to quantify overdiagnosis (8). The principal limitation of this measurement is that it fails to account for the effect of competing lethal morbidities, which are age and tumor-dimension-related (see supplementary) Comparison of the number of cases in the screened vs. control cohorts, after allowing sufficient follow-up for the appearance of most clinically relevant cancers in the controls, is a simple method of estimating its magnitude. Many more cases (47%, 45%, respectively)—almost exclusively stages I–II—were identified in the European LDCT-screened cohorts vs. null controls after an 8–9-year period of follow-up (4,5). Considering their stage and the duration of follow-up, the majority of the excess cases were likely overdiagnosed. The percent of excess cases in the LDCT vs. null controls is twice that in CR vs. null controls—22–24% (3) and 3.5-fold that in LDCT vs. CR—13% (1).
Reduced long-term, postoperative, disease-free survival
Eguchi et al. reported that in patients ≤ 65, 65–75, and ≥ 75 years of age, their intermediate-term (5-year) noncancer cumulative incidence of death were, respectively, 1.8%, 4.9%, and 9.0% (9). Brown et al. (10) compared non-cancer mortality of persons with lung cancer to the U.S. population and found their relative hazard of death to be nearly three-fold the non-cancer death hazard in age and gender matched persons with cancers originating in non-vital organs viz., colon and breast. Sugimura and Yang (11) in a review of long-term survivorship in lung cancer, reported post-resection disease-free relative survival of 60% vs. matched U.S. peers. They pointed out an additional harm: many long-term survivors of lung cancer experienced a marked reduction in their quality of life. We reported a virtually identical (41%) reduction in long-term disease-free survival vs. matched U.S peers in persons who underwent lobectomy for stage I NSCLC. The design of our study differed from the prior two by identifying loss of pulmonary reserve as its primary cause and by identifying a 6-year latency (Figure 1) in the appearance of bulk of the survival deficit (12). A deficit of this magnitude, were it attributable solely to the subjects’ smoking-related comorbidities, would be incompatible with their tolerance of a major surgical procedure and the observation that they experienced little reduction in life expectancy for 6 years following the surgical loss of ≥ 1/9th of their pulmonary reserve.
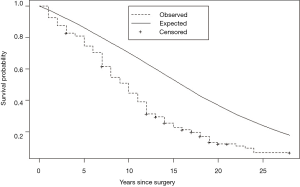
Combined effect of overdiagnosis and reduced long-term disease-free survival
In centers of excellence providing LDCT-screening and surgical management to selected individuals, nearly half the persons with lung cancers will be overdiagnosed. Their life expectancy will be reduced by 40%, many will experience a marked reduction in their quality of life and some will undergo an interventional cascade prompted by incidental findings. For overdiagnosed individuals, there is no potential offsetting benefit. For LDCT screening to be beneficial, it will have to achieve an offsetting reduction in lung cancer mortality that substantially exceeds these harms.
Summary and conclusions
- European LDCT screening trials, employing null controls, have thus far failed to reproduce the benefit reported in the much larger NLST, which employed CR controls. Collectively, they reported worse outcomes in the screened cohorts.
- Centers of excellence screening selected subjects achieved a remarkably low level of surgical mortality. To what extent this will be reproduced in community settings is unknown.
- Long-term survivors of lung cancer will experience a ca. 40% reduction in life expectancy; most of the deficit will appear after 6 years; many will, in addition, experience a marked diminution in their quality of life.
- Valid assessment of all-cause mortality requires longer-term (>6-year) follow-up.
- Nearly half the persons identified with lung cancer by LDCT screening will be overdiagnosed, all of whom will be harmed. For screening to be successful, its benefit in reducing lung cancer and all-cause mortality will have to far outweigh its harms.
Collectively, these observations justify caution in advocating population screening for lung cancer. An additional 5-year analysis of outcome in the NLST would be welcome as will the reports of the collective experience from ongoing European trials.
Supplementary
Some readers may find helpful a brief summary of the derivation and employment of algorithms which we found conceptually useful in addressing overdiagnosis and disease-free lung cancer survival.
Geddes (13) furnished conceptual and empirical evidence that lung cancer growth can be modeled as an exponential function that assumed clonal origin and a constant TVDT. Under these assumptions, the lifetime natural history of LC comprised 40 tumor volume doublings (TVDs) to achieve a lethal diameter of ca. 10 cm. The volume of a sphere is given by (4/3)πr3. Volume doubling therefore requires an increase of the radius by a factor of the cube root of 2 (=1.26). Under the assumption that the clonal cell diameter (D) is 0.001 cm, the number of TVD (x) required to achieve a specified D (y) in cm is given by: tumor D = cell D (cube root of 2)number of TVD, or y = 0.001(1.26)x. Conversion to the logarithmic form simplifies computation: x=ln(1000y)⁄ln(1.26) or ln1000y)⁄0.231 (14). We estimated mean TVDT of stage I NSCLC = 230 days (14). Under these assumptions, the number of TVDs required to achieve a diameter of 0.5, 1.5 and 2.5 cm are, respectively, 26.9, 31.6 and 33.9. To attain a lethal diameter of 10 cm requires 13.1, 8.4 and 6.1 further TVDs corresponding to 8.3, 5.3 and 3.8 years. The duration of exposure to competing lethal comorbidities directly affects the likelihood of overdiagnosis. The implied latency of stage I NSCLC-specific cumulative incidence of death (CID) vs. the immediate onset of non-cancer specific CID accounts for the observation of Eguchi et al: “for up to 2.5 years after resection, in persons ≥65 years old, noncancer-specific CID was higher than lung cancer-specific CID” (15).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- The National Lung Screening Trial Research Team. Results of Initial Low-Dose Computed Tomographic Screening for Lung Cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Oken MM, Hocking WG, Kvale PA, et al. PLCO Project Team. Screening by chest radiograph and lung cancer mortality: the prostate, lung, colorectal, and ovarian (PLCO) randomized trial. JAMA 2011;306:1865-73. [Crossref] [PubMed]
- Reich JM, Kim JS. The national lung screening trial premise of null and chest radiographic control equivalence is open to question. AJR Am J Roentgenol 2015;205:278-9. [Crossref] [PubMed]
- Wille MM, Dirksen A, Haseem A, et al. Results of the randomized Danish Lung Cancer Screening Trial with focus on high-risk profiling. Am J Respir Crit Care Med 2016;193:542-51. [Crossref] [PubMed]
- Infante M, Sestini S, Galeane C, et al. Lung cancer screening with low-dose spiral computerized tomorgraphy: evidence from a pooled analysis of two Italian randomized trials. Eur J Cancer Prev 2017;26:324-9. [Crossref] [PubMed]
- Flores R, Bauer T, Aye R, et al. I-ELCAP Investigators. Balancing curability and unnecessary surgery in the context of computed tomography screening for lung cancer. J Thorac Cardiovasc Surg 2014;147:1619-26. [Crossref] [PubMed]
- Rosen JE, Hancock JG, Kim AW, et al. Predictors of mortality after surgical management of lung cancer in the national cancer database. Ann Thorac Surg 2014;98:1953-60. [Crossref] [PubMed]
- Veronesi G, Maisonneuve P, Bellomi M, et al. Estimating overdiagnosis in low-dose computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2012;157:776-84. [Crossref] [PubMed]
- Eguchi T, Bains S, Lee MC, et al. Impact of increasing age on cause-specific mortality and morbidity in patients with stage I non–small-cell lung cancer: a competing risks analysis. J Clin Oncol 2017;35:281-90. [Crossref] [PubMed]
- Brown BW, Brauner C, Minnotte MC. Noncancer deaths in white adult cancer patients. J Natl Cancer Inst 1993;85:979-87. [Crossref] [PubMed]
- Sugimura H, Yang P. Long-term survivorship in lung cancer: a review. Chest 2006;129:1088-97. [Crossref] [PubMed]
- Reich JM, Kim JS, Asaph JW. Diminished Disease-Free Survival After Lobectomy: Screening Implications. Clin Lung Cancer 2015;16:391-97. [Crossref] [PubMed]
- Geddes DM. The natural history of lung cancer: a review based on rates of tumour growth. Br J Dis Chest 1979;73:1-17. [Crossref] [PubMed]
- Reich JM, Kim JS. Lung cancer growth dynamics. Eur J Radiol 2011;80:e458-61. [Crossref] [PubMed]
- Eguchi T, Bains S, Lee MC, et al. Impact of increasing age on cause-specific mortality and morbidity in patients with stage I non-small-cell lung cancer: a competing risks analysis. J Clin Oncol 2017;35:281-90. [Crossref] [PubMed]


