A neuroanatomical framework for the central modulation of respiratory sensory processing and cough by the periaqueductal grey
An overview of airway sensory processing in the brain
The airways and lungs are innervated by heterogeneous populations of sensory nerve fibers that respond to chemical and mechanical stimulation of the respiratory system (1). Many of these sensory nerve fibers are vagal in origin, derived from one of two distinct clusters of sensory neurons known as the nodose (or inferior) and jugular (or superior) vagal ganglia. In healthy airways, the activation of vagal sensory neurons evokes a wide range of respiratory responses and sensations that are important for the ongoing regulation of breathing, airway clearance and the maintenance of airway patency. These responses depend on well-defined reflex circuits integrated in the brainstem and less well-defined networks within the higher brain. The latter represents an essential component of the more complex behavioral responses that accompany airway stimulation in health and disease occurring through ascending connections with subcortical and cortical brain regions or through the modulation of bulbar reflexes via descending control systems (2,3). In this brief review, we will focus on the higher brain circuits in receipt of airway sensory inputs, with a particular emphasis on a descending modulatory system involving the midbrain periaqueductal grey. This descending system is of interest because it may be capable of potently regulating airway afferent nerve-mediated responses and recent evidence has demonstrated plasticity in descending control in patients with cough hypersensitivity (4). In exploring this topic, we will highlight possible central therapeutic targets for curtailing symptoms of pulmonary disease associated with excessive sensory nerve activity and propose neural mechanisms that might contribute to, or be targets for, inappropriate cough control.
Airway sensory inputs reach the higher brain via multiple ascending circuits. In rodents, at least two ascending pathways have been described. The first is specific for nodose ganglia-derived afferent fibers that synapse in the medullary nucleus of the solitary tract from which projections are sent to pontine and midbrain nuclei (e.g., lateral parabrachial nucleus and locus coeruleus), the hypothalamus (especially the paraventricular nucleus and lateral hypothalamic area), zona incerta, thalamus (mediodorsal and ventral posteromedial nuclei) and limbic brain (amygdala, insula and cingulate cortex) (5). The second ascending pathway is specific for jugular ganglia-derived afferent fibers, which terminate in the medullary paratrigeminal and trigeminal nuclei (rather than the nucleus of the solitary tract) from which ascending projections are sent extensively throughout central somatosensory processing networks including to the thalamus (ventral posterolateral and submedius nuclei) and somatosensory cortices (5). Consistent with this, functional brain imaging studies in humans have demonstrated that multiple central networks process the sensory (stimulus location, intensity and perception) and affective (degree of unpleasantness and emotional valencies) dimensions resultant from airway irritant stimulation (6,7). Whether these circuits in humans originate from distinct afferent neuron subsets innervating the airways, as they do in rodents, is not known. Regardless, these circuits presumably act in concert to promote motor behaviors (like coughing) that help to remove the initiating stimulus and relieve the sensory drive. Superimposed on these core sensorimotor circuits are several brain systems capable of modulating airway sensory processing and/or the resultant motor responses (6-8). This system includes network components that can suppress or facilitate sensorimotor processing (Figure 1) involving the prefrontal cortex (involved in placebo modulation of sensation), the insula cortex and inferior frontal gyrus (part of a fronto paralimbic system needed for motor response inhibition) and midbrain nuclei such as the periaqueductal grey (part of descending control).
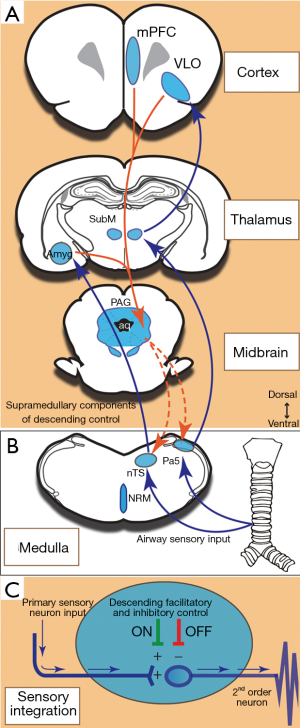
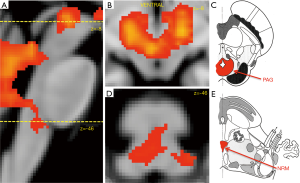
In pulmonary disease, the excessive activation of airway sensory neural pathways is thought to contribute to the development of cough hypersensitivity syndrome, bronchospasm, excessive mucous secretion and the development of unpleasant pulmonary sensations, such as dyspnea and the persistent urge-to-cough. Accordingly, understanding the processes that induce and maintain sensitization should provide a sensible therapeutic pathway for symptom resolution. To this end, many studies have focused on the peripheral inflammatory mediators that are capable of promoting the activation of airway sensors (1). Less attention has focused on the central processes that might contribute to altered sensory neural responses (9). In animal models, pulmonary infections and cigarette smoke exposure enhance synaptic activity between primary sensory neurons and second order neurons in the nucleus of the solitary tract, a phenomenon that has been likened to central sensitization in the spinal cord following inflammatory or neuropathic pain states (9,10). Alternatively, functional brain imaging studies performed in chronic cough patients suggest that enhanced cough sensitivity may coincide with altered neural processing in the networks purported to be involved in modulating sensorimotor processing (4). In particular, activations in the midbrain regions containing the periaqueductal grey and adjacent nucleus cuneiformis are upregulated in cough patients (compared to healthy controls; Figure 2) during the inhalation of irritant stimuli (4). Similar activations are also revealed in conditions of pain hypersensitivity (11). This raises important questions about the role of midbrain processing in the modulation of sensory sensitivity in disease conditions associated with both up and down-regulation of cough control. .
Descending control of sensory processing: the periaqueductal grey and pain
A well described endogenous neural system exists that is capable of modulating primary afferent inputs to second order neurons at the level of the spinal dorsal horn. Often referred to as the ‘analgesia system’ because of its opioid dependent capacity to suppress pain processing, this complex neural network can in fact both suppress and facilitate afferent processing depending on the specific neuronal components recruited. Central to this network is the midbrain periaqueductal grey, which serves to integrate information from multiple (spinal, bulbar and cortical) sources and actuate the modulation of nociceptive processing through neural circuits that exert effects at the level of the spinal dorsal horn.
Stimulation of the periaqueductal grey with opioids or electrical currents evokes profound analgesia in a variety of species (12-18), and deep brain stimulation of the periaqueductal grey has been used therapeutically in patients with intractable pain (19). The periaqueductal grey receives nociceptive inputs from the spinal cord, likely through the parabrachial nucleus (20), and is also subject to descending influences from the cortex and other brain regions. Projections from the periaqueductal grey are widely distributed throughout the hindbrain, notably to pontine and medullary noradrenergic nuclei and the rostral ventromedial medulla, which likely represent the final neural pathways for dorsal horn modulation of nociceptive processing (21). Electrophysiological recordings of neurons in both the periaqueductal grey and rostral ventromedial medulla demonstrate distinct populations of cells defined as either activated or inhibited during the application of peripheral noxious stimuli, appropriately named “ON” and “OFF” cells, respectively. ON cells are presumed to facilitate noxious processing in the spinal cord, while the OFF cells are believed crucial components of the descending inhibitory system because their activity correlates with an inhibition of nociceptive sensory transmission (22,23). Facilitation and suppression are brought about by serotonergic and noradrenergic inputs to primary afferent terminals or dorsal horn interneurons in the spinal cord. The activity of ON cells in both the periaqueductal grey and rostral ventromedial medulla are inhibited by opioidergic inputs whereas OFF cell activity is disinhibited by opioid-dependent inhibition of GABAergic inhibitory inputs. Thus, opioids promote descending inhibition leading to analgesia (24-26). The capacity to facilitate or inhibit sensory processing presumably allows behavioral responses to noxious stimulation to be matched with competing demands (27).
The periaqueductal grey and vagal sensory processing
Conditions of hyperalgesia are associated with an imbalance of inhibitory and facilitatory descending inputs to the spinal cord, effectively reducing the capacity to invoke descending inhibition (28-31). In a functional brain imaging study, we noted that patients with chronic cough displayed increased neural activity in the periaqueductal grey and neighboring nucleus cuneiformis, regions that are similarly activated during pain hypersensitivity (4) (Figure 2). This raises the question of whether vagal afferent processing from the airways is similarly subject to descending modulation from the midbrain periaqueductal grey and associated regions, and whether dysfunction in this system is an important component of cough hypersensitivity. However, airway vagal afferents of course terminate in both the medullary nucleus of the solitary tract and paratrigeminal nucleus (and not the spinal dorsal horn) and as such, it is important to consider whether the periaqueductal grey and/ or rostroventral medial medulla provide functional inputs to these medullary sensory processing nuclei.
The periaqueductal grey has been repeatedly shown to play a role in both respiratory and cardiovascular control, the nature of which depends on the specific subregion under study. For example, both electrical and chemical stimulation in the ventrolateral periaqueductal grey elicit hypotension, vagal bradycardia and facilitate the baroreflex, whereas stimulation in the dorsal region has the opposite effect (32-34). Chemical activation of the dorsolateral periaqueductal grey in rats increases respiratory rate and overall respiratory activity (35,36) and the respiratory effects elicited from the dorsal periaqueductal grey are more prominent from the caudal end of the nucleus, suggesting a rostrocaudal, as well as the dorsoventral, organization (37). However, it is unclear if such effects are mediated by the modulation of medullary sensory processing or through direct influences of premotor neurons in the rostral ventrolateral medullary cardio-respiratory groups. Anatomical tracing studies have demonstrated direct projections from the ventrolateral periaqueductal grey and rostral ventromedial medulla to the nucleus of the solitary tract (38-40), providing an anatomical framework for modulatory control (Figure 1). Functional evidence for descending modulation of vagal afferent processing was demonstrated in an elegant study by Sessle et al. (41) in which stimulation of the cat periaqueductal grey or the nucleus raphe magnus (part of the rostral ventromedial medullary group involved in nociceptive control) significantly inhibited respiration and suppressed vagally-mediated reflex cough and swallow, coinciding with a marked suppression of neuronal activity in the nucleus of the solitary tract. Furthermore, they demonstrated that responses were reversed by the mu-opioid receptor antagonist naloxone, consistent with an activation of the antinociceptive system. Remarkably, stimulation of the periaqueductal grey and raphe magnus in the same animals also inhibited the nociceptive jaw-opening reflex elicited by noxious tooth pulp stimulation, suggesting commonalities between the inhibitory regulation of pain and upper airway sensory evoked responses. Whether descending facilitation can be evoked at the level of the nucleus of the solitary tract was not assessed.
Only recently was it discovered that a population of airway afferents project to the medullary paratrigeminal nucleus (5,42-44) and consequently the possibility of descending modulation of paratrigeminal vagal afferent processing has not been studied. Nevertheless, the periaqueductal grey and the nucleus raphe magnus are important for modulation of trigeminal afferent processing throughout the spinal trigeminal system. For example, electrical stimulation of the periaqueductal grey and nucleus raphe magnus (a component of the rostral ventromedial medulla) inhibited all types of neurons within the trigeminal medullary dorsal horn (45,46) as well as tooth pulp afferent processing within the spinal trigeminal nucleus (47,48). Neuronal responses in the trigeminal oralis region evoked by tooth pulp stimulation were suppressed by both periaqueductal grey and nucleus raphe magnus conditioning stimuli and given that such responses are naloxone-sensitive (49) it is consistent with the activation of the descending antinociceptive system.
Thalamic and cortical regulation of descending control: ‘top-down’ modulation
Descending modulation of sensory processing is clearly well defined, but this raises the question ‘what regulates the periaqueductal grey to drive this descending control’? Although graded sensory stimuli generally result in graded responses (action potentials) in the primary sensory neurons detecting that stimulus, the level of sensation experienced may not correlate with stimulus intensity. Indeed, sensory perception is subject to significant higher brain modulation dependent upon past experiences, stress and anxiety, attention and other complex cognitive processes, and this likely occurs through ‘top-down’ neural pathways arising from the prefrontal cortex and capable of modulating neuronal activity in the midbrain periaqueductal grey (Figure 1).
Studies in both animals and humans have demonstrated the existence of several prefrontal cortical inputs to periaqueductal grey neurons, including from neurons originating in the rostral agranular insula cortex (a small area of cerebral cortex positioned above the rhinal fissure in rodents defined by the absence of cortical layer four), the neighbouring ventrolateral orbital cortex (again adjacent to the rhinal fissure in rodents), as well as from the dorsolateral prefrontal cortex in humans (the medial prefrontal cortex is the homologue in rodents) (50-53). Antinociception can be readily evoked by prefrontal cortex stimulation in rodents, and this is prevented by prior inhibition or lesioning of the ventrolateral periaqueductal grey (54), suggesting that neurons in the periaqueductal grey are central to the prefrontal descending modulation of pain. Transcranial magnetic stimulation of the dorsolateral prefrontal cortex in humans similarly reduces noxious sensations (55) and anatomical tracing studies in rodents confirm the output connectivity of the prefrontal cortex to the periaqueductal grey (56,57).
Prefrontal cortical neurons involved in pain modulation, in turn, receive inputs from a wide variety of central sources. One mechanism of prefrontal cortical regulation involves afferent information being relayed from the spinal dorsal horn to the cortex via an obscure collection of sensory processing neurons in the thalamus known as the submedius nucleus (Figure 1). Best defined in the rats, the submedius nucleus is located close the cerebral midline, ventral to the central medial thalamic nucleus and dorsal to the paraventricular nucleus of the hypothalamus. It is populated by output neurons and local interneurons responsive to noxious stimuli from the viscera, muscles and skin (58-60) and it receives direct nociceptive inputs from neurons in laminar one of the trigeminal nucleus and spinal dorsal horn (61-63). Output neurons of the submedius nucleus project heavily to the prefrontal cortex, including the rostral agranular insula and the ventrolateral orbital cortices. As submedius output neurons are principally glutamatergic in nature, they provide excitatory drive to recipient cortical neurons (64).
The neuropharmacology of these thalamocortical loops has been studied in some detail. The nociceptive-related inputs to the submedius nucleus and prefrontal cortex increases the activity of the output neurons, both directly via the release of glutamate and indirectly by enkephalins that reduce the activity of the local tonically inhibitory GABAergic interneurons, resulting in a net increase in the activity of both submedius nucleus and the recipient cortical neurons (65). This activation pattern can be mimicked by electrical stimulation or exogenously administered glutamate or opioid agonists into either the submedius nucleus or the prefrontal cortex, resulting in a suppression of the behavioral responses to nociception (54,64,66,67). Further functional assessment of the connections showed that inhibiting the prefrontal cortex suppresses the behavioral effects produced by stimulating the submedius nucleus (64), and paradoxically enhanced nociceptive responses consistent with the notion that there is ongoing tonic activity in the descending control network (68). This tonic activity may reflect additional inputs to the submedius nucleus from the raphe (serotonergic) and the reticular thalamus (GABAergic) which provide alternative sources of excitatory and inhibitory (respectively) influence over submedius output neurons, while prefrontal cortical output neuronal activity is facilitated by inputs from raphe (serotonergic) and the ventral tegmental area (dopaminergic) (53,69,70).
‘Top down’ control of visceral sensory processing
There is evidence to suggest that both the prefrontal cortex and submedius nucleus of the thalamus play a role in the regulation of visceral noxious sensory processing. In this regard, the evidence is perhaps strongest for spinal visceral nociceptive pathways, although some evidence also exists for bulbar visceral afferent pathways. In a model of acute visceral pain, both colorectal distension and noxious somatic stimulation (but not innocuous stimuli) modulated the activity of the same neurons in the ventrolateral orbital cortex and submedius nucleus (71-75), indicating that viscero-somatic sensory convergence was common in the nociceptive control system. Furthermore, administration of intravenous morphine dose-dependently attenuated descending control evoked by noxious visceral stimulation similar to the role that opioidergic pathways play in somatic nociception. Electrical stimulation of the submedius nucleus resulted in intensity dependent attenuation of colorectal distension evoked behavioral responses (75,76), while electrical or chemical stimulation (glutamate) of the periaqueductal grey or the rostral ventromedial medulla inhibited the majority of spinal cord neurons activated by colorectal distension in the rat (77,78). Taken together, these data suggest a role of the thalamo-cortico-bulbar descending pathway in the regulation of visceral nociception.
With respect to vagal afferents, vagus nerve stimulation in the cat induced activity in orbital cortex neurons that were also responsive to cutaneous stimuli (79). Consistent with this, electrical stimulation of the orbital gyrus in anesthetized cats acutely suppressed cough evoked by activation of the superior laryngeal nerve (80) whereas electrolytic lesions of the orbital region enhanced the hypoxic ventilator response, albeit not in all animals studied (81). In humans, placebo conditioning substantially reduces the perception of the urge-to-cough associated with inhaled airway irritants, and the magnitude of this inhibition correlates with the degree of activation in the dorsolateral prefrontal cortex (82), comparable to placebo analgesia. Recently, we used transsynaptic anterograde viral tracers in rats to provide anatomical evidence for airway-specific vagal afferent inputs to the submedius nucleus, ventrolateral orbital cortex and medial prefrontal cortex (42-44) suggesting that vagal afferents might similarly modulate thalamo-cortical inputs to the midbrain (Figure 1). In a follow up study, using a genetically modified conditional transsynaptic viral tracing system, we reported that only the airway vagal afferents passing through the paratrigeminal nucleus in the brainstem (i.e., jugular vagal afferents) contributed to this thalamo-cortical circuit (5). Both the submedius nucleus and the ventrolateral orbital cortex were devoid of inputs via the nodose ganglia and the nucleus of the solitary tract.
Inputs from the amygdala represent an alternative pathway for regulating the activity of periaqueductal grey-mediated descending control (Figure 1). Thus, electrical stimulation of the medial or central amygdala elicits antinociception which is inhibited by blockade of the periaqueductal grey (83), indicative of amygdala-evoked nociceptive control during fear and other aversive states. In this regard, it is interesting that ascending vagal sensory inputs from the airways are relayed extensively to the central nucleus of the amygdala (5,42), perhaps representing an alternative control loop for eliciting descending modulation of vagal sensory processing.
Clinical significance and concluding remarks
The central integration that culminates in generating a respiratory sensation and modulating a respiratory behavior is complex in nature. Many respiratory behaviors are not simply reflexes, but are subject to significant regulation by higher brain processes. Human studies using functional brain imaging have identified forebrain and bulbar response patterns that are consistent with descending control of airway sensory processes (6,84), and animal studies have begun to identify their anatomical connectivity with airway sensory pathways (5,43). Whether these circuits are altered in disease remains largely unstudied and whether they are ultimately targetable from a therapeutic standpoint to relieve the symptoms of disease remains to be seen. For example, cough can be both up-regulated (for example in pulmonary disease) and down-regulated (in neurological conditions such as Parkinson’s disease) and it is tempting to speculate that modulation of either the inhibitory or facilitatory descending control networks might provide a therapeutic option for normalising cough in these conditions. Indeed, opioid therapy is the current gold-standard antitussive agent, and likely suppresses cough due to an action within the descending inhibitory control systems of the brain. Additionally, altered activity within the periaqueductal grey and related nuclei accompanies chronic cough (4) and this could conceivably either contribute to the establishment of up-regulated cough states (i.e., enhanced facilitation) or represent the recruitment of descending control mechanisms as a compensatory strategy in an attempt to dampen disordered coughing (i.e., enhanced inhibition). Whether similar plasticity occurs in these networks in conditions of down-regulated cough is not known. Accordingly, a deeper understanding of the role of descending control in disordered cough states is warranted as this may afford novel opportunities for regulating disordered cough in a myriad of diseases.
Acknowledgements
Funding: This research was supported by a grant to A/Profs Mazzone and Farrell from the National Health and Medical Research Council (NHMRC) of Australia (1078943).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mazzone SB, Undem BJ. Vagal Afferent Innervation of the Airways in Health and Disease. Physiol Rev 2016;96:975-1024. [Crossref] [PubMed]
- Driessen AK, Farrell MJ, Mazzone SB, et al. Multiple neural circuits mediating airway sensations: Recent advances in the neurobiology of the urge-to-cough. Respir Physiol Neurobiol 2016;226:115-20. [Crossref] [PubMed]
- Mazzone SB, McGovern AE, Farrell MJ. Endogenous central suppressive mechanisms regulating cough as potential targets for novel antitussive therapies. Curr Opin Pharmacol 2015;22:1-8. [Crossref] [PubMed]
- Ando A, Smallwood D, McMahon M, et al. Neural correlates of cough hypersensitivity in humans: evidence for central sensitisation and dysfunctional inhibitory control. Thorax 2016;71:323-9. [Crossref] [PubMed]
- McGovern AE, Driessen AK, Simmons DG, et al. Distinct brainstem and forebrain circuits receiving tracheal sensory neuron inputs revealed using a novel conditional anterograde transsynaptic viral tracing system. J Neurosci 2015;35:7041-55. [Crossref] [PubMed]
- Farrell MJ, Cole LJ, Chiapoco D, et al. Neural correlates coding stimulus level and perception of capsaicin-evoked urge-to-cough in humans. NeuroImage 2012;61:1324-35. [Crossref] [PubMed]
- Farrell MJ, Koch S, Ando A, et al. Functionally connected brain regions in the network activated during capsaicin inhalation. Hum Brain Mapp 2014;35:5341-55. [Crossref] [PubMed]
- Mazzone SB, Cole LJ, Ando A, et al. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J Neurosci 2011;31:2948-58. [Crossref] [PubMed]
- Driessen AK, McGovern AE, Narula M, et al. Central mechanisms of airway sensation and cough hypersensitivity. Pulm Pharmacol Ther 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Chung KF, McGarvey L, Mazzone SB. Chronic cough as a neuropathic disorder. Lancet Respir Med 2013;1:414-22. [Crossref] [PubMed]
- Zambreanu L, Wise RG, Brooks JC, et al. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain 2005;114:397-407. [Crossref] [PubMed]
- Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science 1969;164:444-5. [Crossref] [PubMed]
- Yeung JC, Yaksh TL, Rudy TA. Concurrent mapping of brain sites for sensitivity to the direct application of morphine and focal electrical stimulation in the production of antinociception in the rat. Pain 1977;4:23-40. [Crossref] [PubMed]
- Deakin JF, Dostrovsky JO. Involvement of the periaqueductal grey matter and spinal 5-hydroxytryptaminergic pathways in morphine analgesia: effects of lesions and 5-hydroxytryptamine depletion. Br J Pharmacol 1978;63:159-65. [Crossref] [PubMed]
- Jones SL, Gebhart GF. Inhibition of spinal nociceptive transmission from the midbrain, pons and medulla in the rat: activation of descending inhibition by morphine, glutamate and electrical stimulation. Brain Res 1988;460:281-96. [Crossref] [PubMed]
- Waters AJ, Lumb BM. Inhibitory effects evoked from both the lateral and ventrolateral periaqueductal grey are selective for the nociceptive responses of rat dorsal horn neurones. Brain Res 1997;752:239-49. [Crossref] [PubMed]
- Lee BH, Park SH, Won R, et al. Antiallodynic effects produced by stimulation of the periaqueductal gray matter in a rat model of neuropathic pain. Neurosci Lett 2000;291:29-32. [Crossref] [PubMed]
- Campion KN, Saville KA, Morgan MM. Relative contribution of the dorsal raphe nucleus and ventrolateral periaqueductal gray to morphine antinociception and tolerance in the rat. Eur J Neurosci 2016;44:2667-72. [Crossref] [PubMed]
- Sims-Williams H, Matthews JC, Talbot PS, et al. Deep brain stimulation of the periaqueductal gray releases endogenous opioids in humans. Neuroimage 2017;146:833-42. [Crossref] [PubMed]
- Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol 2002;87:251-8. [Crossref] [PubMed]
- Ossipov MH. The perception and endogenous modulation of pain. Scientifica (Cairo) 2012;2012:561761. [PubMed]
- Vanegas H, Barbaro NM, Fields HL. Tail-flick related activity in medullospinal neurons. Brain Res 1984;321:135-41. [Crossref] [PubMed]
- Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol 1995;74:1742-59. [PubMed]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 1984;7:309-38. [Crossref] [PubMed]
- Moreau JL, Fields HL. Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Res 1986;397:37-46. [Crossref] [PubMed]
- Heinricher MM, Morgan MM, Tortorici V, et al. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience 1994;63:279-88. [Crossref] [PubMed]
- Fields H. State-dependent opioid control of pain. Nat Rev Neurosci 2004;5:565-75. [Crossref] [PubMed]
- Rahman W, D'Mello R, Dickenson AH. Peripheral nerve injury-induced changes in spinal alpha (2)-adrenoceptor-mediated modulation of mechanically evoked dorsal horn neuronal responses. J Pain 2008;9:350-9. [Crossref] [PubMed]
- Taylor BK. Spinal inhibitory neurotransmission in neuropathic pain. Curr Pain Headache Rep 2009;13:208-14. [Crossref] [PubMed]
- Kimura M, Sakai A, Sakamoto A, et al. Glial cell line-derived neurotrophic factor-mediated enhancement of noradrenergic descending inhibition in the locus coeruleus exerts prolonged analgesia in neuropathic pain. Br J Pharmacol 2015;172:2469-78. [Crossref] [PubMed]
- Huang YJ, Lee KH, Murphy L, et al. Acute spinal cord injury (SCI) transforms how GABA affects nociceptive sensitization. Exp Neurol 2016;285:82-95. [Crossref] [PubMed]
- Carrive P, Bandler R. Viscerotopic organization of neurons subserving hypotensive reactions within the midbrain periaqueductal grey: a correlative functional and anatomical study. Brain Res 1991;541:206-15. [Crossref] [PubMed]
- Lovick TA. Inhibitory modulation of the cardiovascular defence response by the ventrolateral periaqueductal grey matter in rats. Exp Brain Res 1992;89:133-9. [Crossref] [PubMed]
- Inui K, Murase S, Nosaka S. Facilitation of the arterial baroreflex by the ventrolateral part of the midbrain periaqueductal grey matter in rats. J Physiol 1994;477:89-101. [Crossref] [PubMed]
- Horiuchi J, McDowall LM, Dampney RA. Vasomotor and respiratory responses evoked from the dorsolateral periaqueductal grey are mediated by the dorsomedial hypothalamus. J Physiol 2009;587:5149-62. [Crossref] [PubMed]
- Iigaya K, Horiuchi J, McDowall LM, et al. Topographical specificity of regulation of respiratory and renal sympathetic activity by the midbrain dorsolateral periaqueductal gray. Am J Physiol Regul Integr Comp Physiol 2010;299:R853-61. [Crossref] [PubMed]
- Zhang W, Hayward LF, Davenport PW. Respiratory responses elicited by rostral versus caudal dorsal periaqueductal gray stimulation in rats. Auton Neurosci 2007;134:45-54. [Crossref] [PubMed]
- Farkas E, Jansen AS, Loewy AD. Periaqueductal gray matter projection to vagal preganglionic neurons and the nucleus tractus solitarius. Brain Res 1997;764:257-61. [Crossref] [PubMed]
- Babic T, Ciriello J. Medullary and spinal cord projections from cardiovascular responsive sites in the rostral ventromedial medulla. J Comp Neurol 2004;469:391-412. [Crossref] [PubMed]
- Babic T, de Oliveira CV, Ciriello J. Collateral axonal projections from rostral ventromedial medullary nitric oxide synthase containing neurons to brainstem autonomic sites. Brain Res 2008;1211:44-56. [Crossref] [PubMed]
- Sessle BJ, Ball GJ, Lucier GE. Suppressive influences from periaqueductal gray and nucleus raphe magnus on respiration and related reflex activities and on solitary tract neurons, and effect of naloxone. Brain Res 1981;216:145-61. [Crossref] [PubMed]
- McGovern AE, Davis-Poynter N, Farrell MJ, et al. Transneuronal tracing of airways-related sensory circuitry using herpes simplex virus 1, strain H129. Neuroscience 2012;207:148-66. [Crossref] [PubMed]
- McGovern AE, Davis-Poynter N, Rakoczy J, et al. Anterograde neuronal circuit tracing using a genetically modified herpes simplex virus expressing EGFP. J Neurosci Methods 2012;209:158-67. [Crossref] [PubMed]
- McGovern AE, Davis-Poynter N, Yang SK, et al. Evidence for multiple sensory circuits in the brain arising from the respiratory system: an anterograde viral tract tracing study in rodents. Brain Struct Funct 2015;220:3683-99. [Crossref] [PubMed]
- Dostrovsky JO, Shah Y, Gray BG. Descending inhibitory influences from periaqueductal gray, nucleus raphe magnus, and adjacent reticular formation. II. Effects on medullary dorsal horn nociceptive and nonnociceptive neurons. J Neurophysiol 1983;49:948-60. [PubMed]
- Chiang CY, Hu JW, Sessle BJ. Parabrachial area and nucleus raphe magnus-induced modulation of nociceptive and nonnociceptive trigeminal subnucleus caudalis neurons activated by cutaneous or deep inputs. J Neurophysiol 1994;71:2430-45. [PubMed]
- Yokota T, Hashimoto S. Periaqueductal gray and tooth pulp afferent interaction on units in caudal medulla oblongata. Brain Res 1976;117:508-12. [Crossref] [PubMed]
- Chiang CY, Sessle BJ, Hu JW. Parabrachial area and nucleus raphe magnus-induced modulation of electrically evoked trigeminal subnucleus caudalis neuronal responses to cutaneous or deep A-fiber and C-fiber inputs in rats. Pain 1995;62:61-8. [Crossref] [PubMed]
- Sessle BJ, Hu JW. Raphe-induced suppression of the jaw-opening reflex and single neurons in trigeminal subnucleus oralis, and influence of naloxone and subnucleus caudalis. Pain 1981;10:19-36. [Crossref] [PubMed]
- Hardy SGP, Leichnetz GR. Frontal cortex projections to the periaqueductal gray in the rat: a retrograde and orthograde horseradish peroxidase study. Neurosci Lett 1981;23:13-7. [Crossref] [PubMed]
- Floyd NS, Price JL, Ferry AT, et al. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J Comp Neurol 2000;422:556-78. [Crossref] [PubMed]
- Hadjipavlou G, Dunckley P, Behrens TE, et al. Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: a diffusion tensor imaging study in healthy controls. Pain 2006;123:169-78. [Crossref] [PubMed]
- Huo FQ, Chen T, Lv BC, et al. Synaptic connections between GABAergic elements and serotonergic terminals or projecting neurons in the ventrolateral orbital cortex. Cerebral Cortex 2009;19:1263-72. [Crossref] [PubMed]
- Zhang YQ, Tang JS, Yuan B, et al. Inhibitory effects of electrically evoked activation of ventrolateral orbital cortex on the tail-flick reflex are mediated by periaqueductal gray in rats. Pain 1997;72:127-35. [Crossref] [PubMed]
- Taylor JJ, Borckardt JJ, George MS. Endogenous opioids mediate left dorsolateral prefrontal cortex rTMS-induced analgesia. Pain 2012;153:1219-25. [Crossref] [PubMed]
- Coffield JA, Bowen KK, Miletic V. Retrograde tracing of projections between the nucleus submedius, the ventrolateral orbital cortex, and the midbrain in the rat. J Comp Neurol 1992;321:488-99. [Crossref] [PubMed]
- Jasmin L, Burkey AR, Granato A, et al. Rostral agranular insular cortex and pain areas of the central nervous system: a tract-tracing study in the rat. J Comp Neurol 2004;468:425-40. [Crossref] [PubMed]
- Dostrovsky JO, Guilbaud G. Noxious stimuli excite neurons in nucleus submedius of the normal and arthritic rat. Brain Res 1988;460:269-80. [Crossref] [PubMed]
- Dostrovsky JO, Guilbaud G. Nociceptive responses in medial thalamus of the normal and arthritic rat. Pain 1990;40:93-104. [Crossref] [PubMed]
- Kawakita K, Dostrovsky JO, Tang JS, et al. Responses of neurons in the rat thalamic nucleus submedius to cutaneous, muscle and visceral nociceptive stimuli. Pain 1993;55:327-38. [Crossref] [PubMed]
- Dado RJ, Giesler GJ Jr. Afferent input to nucleus submedius in rats: retrograde labeling of neurons in the spinal cord and caudal medulla. J Neurosci 1990;10:2672-86. [PubMed]
- Blomqvist A, Ericson AC, Broman J, et al. Electron microscopic identification of lamina I axon terminations in the nucleus submedius of the cat thalamus. Brain Res 1992;585:425-30. [Crossref] [PubMed]
- Yoshida A, Dostrovsky JO, Chiang CY. The afferent and efferent connections of the nucleus submedius in the rat. J Comp Neurol 1992;324:115-33. [Crossref] [PubMed]
- Zhang S, Tang JS, Yuan B, et al. Inhibitory effects of glutamate-induced activation of thalamic nucleus submedius are mediated by ventrolateral orbital cortex and periaqueductal gray in rats. Eur J Pain 1998;2:153-63. [Crossref] [PubMed]
- Sherman SM, Guillery RW. Exploring the Thalamus. San Diego: Academic Press, 2001.
- Dong YF, Tang JS, Yuan B, et al. Morphine applied to the thalamic nucleus submedius produces a naloxone reversible antinociceptive effect in the rat. Neurosci Lett 1999;271:17-20. [Crossref] [PubMed]
- Yang ZJ, Tang JS, Jia H. Morphine microinjections into the rat nucleus submedius depress nociceptive behavior in the formalin test. Neurosci Lett 2002;328:141-4. [Crossref] [PubMed]
- Xie YF, Wang J, Huo FQ, et al. Mu but not delta and kappa opioid receptor involvement in ventrolateral orbital cortex opioid-evoked antinociception in formalin test rats. Neuroscience 2004;126:717-26. [Crossref] [PubMed]
- Matsuzaki S, Takada M, Li YQ, et al. Serotoninergic projections from the dorsal raphe nucleus to the nucleus submedius in the rat and cat. Neuroscience 1993;55:403-16. [Crossref] [PubMed]
- Wang J, Huo FQ, Li YQ, et al. Thalamic nucleus submedius receives GABAergic projection from thalamic reticular nucleus in the rat. Neuroscience 2005;134:515-23. [Crossref] [PubMed]
- Kawakita K, Sumiya E, Murase K, et al. Response characteristics of nucleus submedius neurons to colo-rectal distension in the rat. Neurosci Res 1997;28:59-66. [Crossref] [PubMed]
- Follett KA, Dirks B. Responses of neurons in ventrolateral orbital cortex to noxious visceral stimulation in the rat. Brain Res 1995;669:157-62. [Crossref] [PubMed]
- Yang SW, Follett KA, Piper JG, et al. The effect of morphine on responses of mediodorsal thalamic nuclei and nucleus submedius neurons to colorectal distension in the rat. Brain Res 1998;779:41-52. [Crossref] [PubMed]
- Yang S, Follett KA. The effect of morphine on responses of ventrolateral orbital cortex (VLO) neurons to colorectal distension in the rat. Brain Res 1998;808:101-5. [Crossref] [PubMed]
- Yang Sw, Follett KA. Electrical stimulation of thalamic Nucleus Submedius inhibits responses of spinal dorsal horn neurons to colorectal distension in the rat. Brain Res Bull 2003;59:413-20. [Crossref] [PubMed]
- Sumiya E, Kawakita K. Inhibitory effects of acupuncture manipulation and focal electrical stimulation of the nucleus submedius on a viscerosomatic reflex in anesthetized rats. Jpn J Physiol 1997;47:121-30. [Crossref] [PubMed]
- Ness TJ, Gebhart GF. Quantitative comparison of inhibition of visceral and cutaneous spinal nociceptive transmission from the midbrain and medulla in the rat. J Neurophysiol 1987;58:850-65. [PubMed]
- Okada K, Murase K, Kawakita K. Effects of electrical stimulation of thalamic nucleus submedius and periaqueductal gray on the visceral nociceptive responses of spinal dorsal horn neurons in the rat. Brain Res 1999;834:112-21. [Crossref] [PubMed]
- Hardstaff VE, Jagadeesh P, Newman PP. Activity evoked in the orbital cortex from splanchnic, vagal and cutaneous afferents. J Physiol 1973;231:16P-18P. [PubMed]
- Kasé Y, Kito G, Miyata T, et al. Influence of cerebral cortex stimulation upon cough-like spasmodic expiratory response (SER) and cough in the cat. Brain Res 1984;306:293-8. [Crossref] [PubMed]
- Tenney SM, St John WM. Is there localized cerebral cortical influence on hypoxic ventilatory response? Respir Physiol 1980;41:227-32. [Crossref] [PubMed]
- Leech J, Mazzone SB, Farrell MJ. Brain activity associated with placebo suppression of the urge-to-cough in humans. Am J Respir Crit Care Med 2013;188:1069-75. [Crossref] [PubMed]
- Oliveira MA, Prado WA. Role of PAG in the antinociception evoked from the medial or central amygdala in rats. Brain Res Bull 2001;54:55-63. [Crossref] [PubMed]
- Mazzone SB, McLennan L, McGovern AE, et al. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Resp Crit Care Med 2007;176:327-32. [Crossref] [PubMed]


