Lymph node involvement according to lung adenocarcinoma subtypes: lymph node involvement is influenced by lung adenocarcinoma subtypes
Introduction
Lung cancer is the leading cause of cancer-related deaths in the world (1-3). Adenocarcinoma is the most common histological type of non-small cell lung cancer (1,4). The lung adenocarcinoma classification is proposed by the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society (IASLC/ATS/ERS) (1,5). The lung adenocarcinoma classification proposed by IASLC/ATS/ERS divided invasive adenocarcinoma into six groups based on comprehensive histologic subtyping by the predominant pattern: lepidic, acinar, papillary, micropapillary, solid, and variant (1,5). Recent studies showed that the invasive adenocarcinoma subtypes were significantly associated with recurrence and survival (3,4,6). Micropapillary and solid predominant subtypes are known to be poorer prognostic (7-10); however, the reason for this remains unclear (4,11). The most valuable prognostic factor in non-small cell lung cancer is the pathological stage (6). Lymph node (LN) involvement is a key factor for survival; N2 disease is not considered local, and may be even systemic (12). It is uncertain whether LN involvement is influenced by the lung adenocarcinoma subtype (6,13). In the present study, to find out the reason for the difference in prognoses according to the invasive adenocarcinoma subtypes, we investigated the influence of LN involvement by the constituent histologic subtypes in the tumor, analyzed its influence on survival outcomes, and predicted the predominant histologic subtypes in LN according to the component ratio of the predominant histologic subtype in the tumor.
Methods
Patients and methods
A total of consecutive 97 patients who underwent surgical resection for lung adenocarcinoma between February 2009 and December 2015 were included into the present study and retrospectively analyzed. All patients underwent anatomical resection (lobectomy with/without segmentectomy or wedge resection) with systematic LN dissection. Cancer stage was determined using the seventh American Joint Committee on Cancer staging system. All histological typing was determined as invasive adenocarcinoma using the lung adenocarcinoma multidisciplinary classification proposed by IASLC/ATS/ERS. Inclusion criteria were anatomical resection with standard mediastinal LN dissection, curative intent, lung adenocarcinoma histology, no neoadjuvant or previous chemoradiation therapy before surgery, and LN involvement (N1 and N2 disease). Exclusion criteria were postoperative death within one month due to postoperative complications, other concurrent primary cancer, and palliation or salvage treatment. Preoperative evaluations consisted of chest computed tomography (CT), positron emission tomography (PET)-CT, bone scan, brain magnetic resonance imaging (MRI), endobronchial ultrasound (EBUS), and mediastinoscopy if needed. Adjuvant therapies were performed according to the National Comprehensive Cancer Network guidelines and the recommendations of a multidisciplinary team who reviewed cancer status, resectability or operability, and patient condition. Recurrence or metastasis was diagnosed based on imaging findings including PET-CT, brain MRI, and bone scan, or pathological confirmation when clinically feasible. Mediastinoscopy or EBUS biopsy was employed in cases with maximum standardized uptake value ≥2.5 and size ≥1 cm on PET-CT scan. Three thoracic surgeons performed video-assisted thoracoscopic surgery or conventional thoracotomy with standard mediastinal LN dissection depending on cancer status and patient condition. We analyzed the associations between tumor and LN histological subtypes to investigate the influence of LN involvement by constituent tumor subtypes, disease-free survival (DFS) according to predominant histologic subtype in tumor and LN to investigate its influence on survival outcomes, and predicted the predominant histologic subtypes in LN using the component ratio of the predominant histologic subtype in the tumor.
Histopathological evaluations
All specimens were fixed with formalin and histopathological findings were analyzed using immunohistochemistry staining of single (for LN) and multiple (for tumor) sections. Predominance on LN was analyzed in each metastatic LN. All slides were independently evaluated by two expert pathologists. Evaluation was performed according to the lung adenocarcinoma multidisciplinary classification proposed by IASLC/ATS/ERS; the histological component ratio in tumor and LN was reported in 5% increments. The predominant histologic subtype was defined as the subtype with the largest histological component ratio. The largest number of the sort of the component histological subtype in each slide of tumor (several sections) and LN (single section) was reported to compare the constituent histologic subtypes between tumor and LN.
Statistical considerations and study approval
As our data did not have a normal distribution, all data were analyzed using nonparametric statistical methods. Comparisons between groups were evaluated using the Mann-Whitney U test and the Wilcoxon test. The Chi-squared test or the Fisher’s exact tests were used to evaluate the associations of constituent histologic subtypes between the tumor and LN. The Spearman test was used to evaluate correlation between continuous variables, and the Kaplan-Meier survival estimations were compared with the log-rank tests to identify differences in survival across strata. The propensity score matching method was used to overcome the heterogeneity of data. The receiver operating characteristic (ROC) analysis was conducted to predict the predominant histologic subtype in LN using the component ratio of the predominant histologic subtype in the tumor. The Statistical Package of Social Sciences version 22.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses. A P value <0.05 was considered statistically significant. This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (IRB approval number: KC16RIS0946).
Results
The present study included consecutive 97 patients (male 55, female 42; mean age 63.3±9.1 years) who underwent anatomical and curative surgery for lung adenocarcinoma from February 2009 and December 2015. Surgery methods were lobectomy (n=88), lobectomy with wedge resection (n=3), lobectomy with segmentectomy (n=3), and bilobectomy (n=3). Final pathologic stages were IIa (n=42), IIb (n=1), IIIa (n=43), IIIb (n=2), and IV (n=9). The number of cases with N1 and N2 disease was 50 and 47, respectively. All histologies of lung cancer were invasive lung adenocarcinoma. The number of predominant histologic subtype in tumor was lepidic (n=4), acinar (n=59), micropapillary (n=6), papillary (n=12), solid (n=13), and variant (n=3). The number of predominant histologic subtypes in LN was lepidic (n=0), acinar (n=60), micropapillary (n=9), papillary (n=9), solid (n=16), and variant (n=3). The majority of predominant histologic subtypes were acinar and papillary subtypes (tumor 73.2%, LN 71.1%). The mean observation period was 28.8 (±18.1) months. The overall clinico-pathological characteristics for patient population are in Table 1.
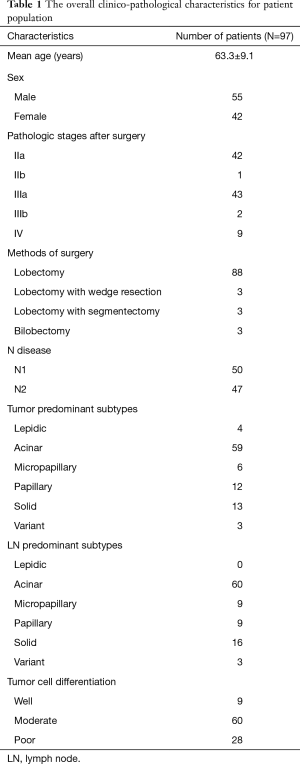
Full table
Comparison of the constituent and the predominant histologic subtypes between tumor and LN
The tumors consisted of a mixture of one to six histologic subtypes and LN consisted of a mixture of one to four histologic subtypes. The tumors had significantly more constituent histologic subtypes than LN (P<0.001) (Table 2). We also investigated whether the constituent and predominant histologic subtypes between tumor and LNs are the same. The constituent histologic subtypes between the tumor and LN were the same in only 15 of 97 cases. The predominant histologic subtype was the same between tumors and LNs in 48 of 97 cases. In order to analyze the associations of the predominant histologic subtypes between the tumor and LN, we divided the six lung adenocarcinoma subtypes into two groups according to known prognostic characteristics (group A: lepidic, acinar, papillary, and variant subtype; group B: micropapillary and solid subtype). The tumors consisted of 78 group A and 19 group B, whereas LN consisted of 72 group A and 25 group B; 20 out of the total 97 cases had different predominant histologic subtype groups between the tumors and LN (transposition from group A into group B was found in 13 cases and transposition from group B into group A was found in 7 cases) and 77 cases had the same predominant histologic subtype groups between the tumors and LN (65 cases with group A and 12 with group B). In conclusion, LN involvement was significantly more enhanced by group B than by group A (all P<0.001, N1 P=0.015, N2 P=0.012) (Table 3).
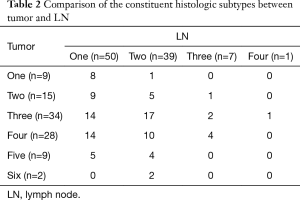
Full table
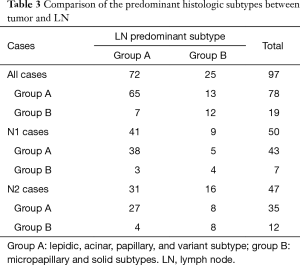
Full table
Association between the predominant histologic subtypes and the cancer progression
We investigated the association between the predominant histologic subtypes and the cancer progression (the pathological stage and the tumor differentiation grade). Micropapillary and solid Predominant subtype were common in more advanced cases; however, this was significant only for tumor cell differentiation grade (tumor P<0.001, LN P=0.001).
DFS according to the predominant histologic subtypes in the tumor and LN
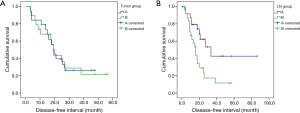
Since the sample size and the number of cancer-related deaths was small and various factors after recurrence might have influenced overall survival. Therefore, we analyzed the DFS using recurrence as a primary endpoint instead of overall survival analysis to clarify the difference in prognosis. The propensity score matching was used to overcome the heterogeneity of data between group A and group B (Table 4). Regarding the predominant histologic subtype in the tumor, there was no significant difference in DFS between group A and group B (P=0.918); however, regarding the predominant histologic subtype in LN, group A showed significantly higher DFS than group B (P=0.010) (Figure 1).
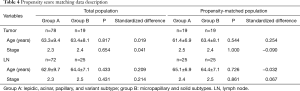
Full table
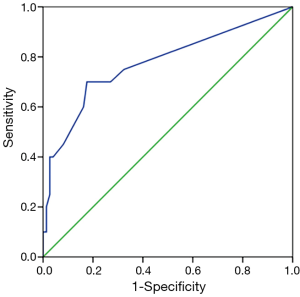
Receiver operating curve analysis for prediction of the predominant histologic subtype in LN using the component ratio of the predominant histologic subtype in the tumor
Among the six predominant histologic subtypes in the tumor, only the solid predominant subtype had a significant cutoff value for the prediction of the predominant histologic subtypes in LN using the predominant histologic subtype component ratio in the tumor (cutoff value 12.5%, sensitivity 70.0%, specificity 82.4%, area under curve 0.775, P<0.001) (Figure 2). The other predominant histologic subtypes in the tumor had no significant cutoff value.
Association of the component ratio of the predominant histologic subtype between the tumors and LN
We investigated the association of the component ratio of the predominant histologic subtype between the tumor and LN in cases with the same the predominant histologic subtype between the tumor and LN. The component ratio of the predominant histologic subtype in LN was significantly higher than in the tumor (LN 85.9%±18.1% vs. tumor 59.4%±27.4%, P<0.001). When cases classified into group A and group B, we similarly found that the component ratio of the predominant histologic subtype in LN was significantly higher than in the tumor (LN 91.6%±12.3% vs. tumor 76.2%±29.2%, P<0.001).
Epidermal growth factor receptor (EGFR) mutation according to the predominant histologic subtypes
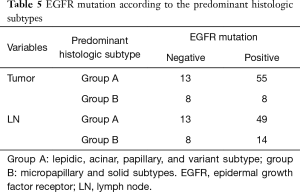
A total of 84 out of 97 cases received the EGFR mutation test (21 negative for EGFR and 63 positive for EGFR). Group A had a significantly higher incidence of EGFR mutation than group B in the tumor. Group A also had a higher incidence of EGFR mutation than group B in LN, however, there was not significant (P=0.251) (Table 5).
Full table
Discussion
Adenocarcinoma is the most common histologic subtype of non-small cell lung cancer (1,4). The standard classification divides invasive adenocarcinoma into the six subtypes on the basis of comprehensive histologic subtyping by predominant pattern: lepidic, acinar, papillary, micropapillary, solid, and variant (1,5). Some recent studies showed that the histological lung adenocarcinoma subtypes proposed by the IASLC/ATS/ERS were associated with prognosis after surgical resection (3,4,6). Lepidic predominant subtype is known to result in better prognosis compared to other histologic subtypes (2). In contrast, micropapillary and solid predominant subtypes are known to result in poorer prognosis (7-10). However, the reason for these differences remains unclear (4,11). Precise diagnosis of LN involvement is essential for management because LN involvement is significantly associated with prognosis (12). In spite of advances in LN evaluation, such as EBUS, mediastinoscopy, and PET-CT, it is not easy to accurately diagnose LN status (12). It is also uncertain whether LN involvement is influenced by the lung adenocarcinoma subtypes (6,13). In the present study, to find out the reason of the difference in prognosis according to the subtypes, especially poorer prognosis in case of micropapillary and solid subtypes, we investigated the influence of LN involvement by the constituent histologic subtypes in the tumor and analyzed its influence on survival outcomes.
As in previous studies, the present study showed that acinar and papillary predominant subtypes occupied the majority of the constituent histologic subtype (14-16). The constituent histologic subtypes in the tumor were not equal to those in LN and the predominant histologic subtype in the tumor was not the same as that in LN. The tumors had significantly more constituent histologic subtypes than LNs. The component ratio of the predominant histologic subtype in LN was significantly higher than that in the tumor. Micropapillary and solid predominant subtypes were significantly more prone to LN involvement. Above findings indicated that if the tumor contains more aggressive component histologic subtypes, LN metastases will be enhanced by them. In addition, the present study showed that micropapillary and solid predominant subtypes in LN had poorer prognosis than other subtypes. These findings indicated that the constituent histologic subtypes differed in terms of biological invasion and that micropapillary and solid predominant subtypes were more aggressive and more prone to LN metastasis. We attributed these findings to the premise that LN involvement is influenced by lung adenocarcinoma histologic subtype. In addition, lepidic predominant subtype in the tumor was not found in LNs, providing an explanation for better prognosis in lepidic predominant subtype compared to other subtypes (14,15).
Interestingly, the present study, unlike other studies, showed no significant difference in DFS according to the predominant histologic subtypes in the tumor. However, micropapillary and solid predominant subtypes in LN had a significantly lower DFS than other subtypes (4,13). This discrepancy was assumed to reflect the small sample size and the fact that cases consisted of only N1-2 disease. In cases with LN involvement, the predominant histologic subtype in LN as well as the pathological stage was considered to be more important for prognosis than the predominant tumor subtype. In addition, a previous study reported that the predominant subtype in tumor, but not that of LN, determines outcomes in patients with lung adenocarcinoma with LN metastases (17). However, other subtypes (lepidic and variant type) were not included and the pathological stage was not considered. The prognosis is basically based on the cancer stage.
We also investigated the possibility of predicting the predominant histologic subtype in LN using the component ratio of the predominant histologic subtype in the tumor. We found that solid predominant subtype in the tumor had a significant cutoff value for predicting the predominant histologic subtype in LN, whereas other subtypes were not significantly predictive. Micropapillary predominant subtype in the tumor showed some potential for prediction but this was not statistically significant probably due to the small sample size.
The predominant histologic subtypes seemed to be associated with the cancer progression. However, this was statistically significant only for the tumor cell differentiation grade, probably due to the small sample size (6). These findings may partly explain why micropapillary and solid predominant subtypes seemed to be associated with more advanced disease status (10).
Like previous studies, the incidence of EGFR mutation in solid and micropapillary predominant subtypes was lower than other subtypes, which was considered to be associated with poorer prognosis in solid and micropapillary predominant subtypes (11,14,16,18).
This study has several limitations, including its retrospective single-center design; lack of ethnic diversity; nonrandomized, heterogeneous data; selection bias; and small sample size. Because routine health screening in Korea has become popular and is covered by the national health insurance, surgical cases are mostly in the early stage and there are relatively few cases with LN involvement and no neoadjuvant therapy. As the small number of cases and the heterogeneity of data might have limited the value of the study findings, we used the propensity score matching method to overcome bias.
Findings from the present study should be confirmed with large scaled, prospective, randomized studies for better management and prognosis. To the best our knowledge, the present study is the first to find out a possible reason of the difference in prognosis according to the constituent histologic subtypes through the influence of LN involvement by the predominant histologic subtypes in cases with LN involvement. Because the analysis of invasive adenocarcinoma subtypes can be implemented by routine pathology evaluation at no additional cost, pathology reports should include invasive adenocarcinoma component subtype ratio in LN to help plan the management strategy, especially in cases with LN involvement (5).
Conclusions
The present presented a possible reason of discrepancies in outcomes according to the lung adenocarcinoma constituent subtypes. Micropapillary and solid predominant subtypes had poorer prognosis than other subtypes, which might be explained by increased LN involvement. Further prospective and randomized trials are needed to validate this finding.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (IRB Approval number: KC16RIS0946) and written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Yim J, Zhu LC, Chiriboga L, et al. Histologic features are important prognostic indicators in early stages lung adenocarcinomas. Mod Pathol 2007;20:233-41. [Crossref] [PubMed]
- Zhang J, Wu J, Tan Q, et al. Why do pathological stage IA lung adenocarcinomas vary from prognosis? a clinicopathologic study of 176 patients with pathological stage IA lung adenocarcinoma based on the IASLC/ATS/ERS classification. J Thorac Oncol 2013;8:1196-202. [Crossref] [PubMed]
- Hung JJ, Jeng WJ, Chou TY, et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg 2013;258:1079-86. [Crossref] [PubMed]
- Tang Y, He Z, Zhu Q, et al. The 2011 IASLC/ATS/ERS pulmonary adenocarcinoma classification: a landmark in personalized medicine for lung cancer management. J Thorac Dis 2014;6:S589-96. [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [Crossref] [PubMed]
- Amin MB, Tamboli P, Merchant SH, et al. Micropapillary component in lung adenocarcinoma: a distinctive histologic feature with possible prognostic significance. Am J Surg Pathol 2002;26:358-64. [Crossref] [PubMed]
- Sakao Y, Miyamoto H, Sakuraba M, et al. Prognostic significance of a histologic subtype in small adenocarcinoma of the lung: the impact of nonbronchioloalveolar carcinoma components. Ann Thorac Surg 2007;83:209-14. [Crossref] [PubMed]
- Ohtaki Y, Yoshida J, Ishii G, et al. Prognostic significance of a solid component in pulmonary adenocarcinoma. Ann Thorac Surg 2011;91:1051-7. [Crossref] [PubMed]
- Cha MJ, Lee HY, Lee KS, et al. Micropapillary and solid subtypes of invasive lung adenocarcinoma: clinical predictors of histopathology and outcome. J Thorac Cardiovasc Surg 2014;147:921-928.e2. [Crossref] [PubMed]
- Clay TD, Do H, Sundararajan V, et al. The clinical relevance of pathologic subtypes in metastatic lung adenocarcinoma. J Thorac Oncol 2014;9:654-63. [Crossref] [PubMed]
- Moon Y, Kim KS, Lee KY, et al. Clinicopathologic Factors Associated With Occult Lymph Node Metastasis in Patients With Clinically Diagnosed N0 Lung Adenocarcinoma. Ann Thorac Surg 2016;101:1928-35. [Crossref] [PubMed]
- Yu Y, Jian H, Shen L, et al. Lymph node involvement influenced by lung adenocarcinoma subtypes in tumor size ≤3 cm disease: A study of 2268 cases. Eur J Surg Oncol 2016;42:1714-1719. [Crossref] [PubMed]
- Lee MC, Kadota K, Buitrago D, et al. Implementing the new IASLC/ATS/ERS classification of lung adenocarcinomas: results from international and Chinese cohorts. J Thorac Dis 2014;6:S568-80. [PubMed]
- Sakurai H, Asamura H, Miyaoka E, et al. Differences in the prognosis of resected lung adenocarcinoma according to the histological subtype: a retrospective analysis of Japanese lung cancer registry data. Eur J Cardiothorac Surg 2014;45:100-7. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. The correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac Surg 2014;98:453-8. [Crossref] [PubMed]
- Suda K, Sato K, Shimizu S, et al. Prognostic implication of predominant histologic subtypes of lymph node metastases in surgically resected lung adenocarcinoma. Biomed Res Int 2014;2014:645681.
- Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 2013;8:52-61. [Crossref] [PubMed]


