Uniportal video-assisted lobectomy through a posterior approach
Introduction
Over the years, uniportal video-assisted thoracoscopic surgery (VATS) has become an alternative strategy to standard multi-port VATS lobectomy for performing minor and major thoracic surgery procedures. The standard technique of uniportal VATS involves an anterior 3–5 cm incision performed through the IV–V intercostal space at the level of the posterior axillary line and surgeon stands in front of the patients (1-4). Herein, we reported our experience with uniportal VATS for carrying out anatomical resections as lobectomy using a posterior rather than the standard anterior approach.
Study design
A written signed informed consent for the operation was obtained in all cases. Patients were aware on the possibility of conversion, if required, and that their data could be used for scientific purpose only. Currently, uniportal VATS lobectomy is a standard procedure performed worldwide in the management of lung cancer; in addition the posterior approach for VATS was also previously reported. Thus, no specific approval by Institutional Review Board was required. In the present study, all patients received the same surgical procedure and post-operative treatments without any type of randomization; the clinical data were prospectively registered in a data base and then retrospectively analyzed. Thus, it was not registered as Randomized Control Trial (RCT).
In addition to clinical outcome, we also evaluated our learning curve through the comparison of the post-operative time and the number of the procedure performed, and the post-operative pain through the comparison of Visual Analogue Score (VAS) ranging from 0 (absence of pain) to 10 (maximal pain) score registered at 12, 24, 36 and 48 post-operative hours and at discharge.
Surgical technique
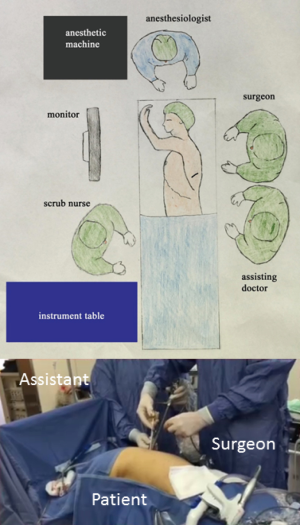
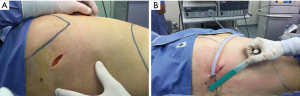
The operative setup for our technique is the same of standard anterior uniportal VATS. The procedure is performed under general anesthesia with single-lung lung ventilation obtained with standard dual-lumen endotracheal tube. The patient is placed in the lateral decubitus position as for standard thoracotomy with slight flexion of the table to allow splaying of the ribs also without the use of rib-spreading. The surgeon and assistant stand at back side of patient while the scrub-nurse and the other assistant stand anteriorly to the patient (Figure 1). As previously reported (5), 10 mL of Bupivacaine is injected at the level of surgical site five minutes before surgical incision to obtain a pre-emptive analgesia effect. Then, a 4–6 cm skin incision is performed through the auscultatory triangle at the level of the fifth intercostal space irrespective of the lobe to be removed (Figure 2). The camera stands in the lower part of the incision and the working instruments above it. However, the position of the camera and working instruments as stapler could change during the different surgical maneuvers (i.e., section of bronchus or vessels) to avoid any conflict. After identification of hilar structures, the dissection proceeds according to the lobe to be resected as follows.
Left upper lobectomy (LUL)
The posterior superior part of the oblique fissure is sectioned with the harmonic scalpel or with an endostapler as convenient. The arterial branches to the left upper lobe including the anterior (A3), the apical-posterior (A1 + A2), and the lingular (A4 + A5) segmental arteries are isolated and either sequentially ligated manually or cut with an endostapler, as convenient. The knots are effected extra-corporeally and pushed in place with a standard needle holder. Then, the division of the anterior part of the oblique fissure is completed, the upper pulmonary vein is exposed and sectioned with a stapler. During this maneuver, care should be taken to separate the superior pulmonary vein from the anterior surface of the bronchus. Finally, the upper left bronchus is mechanically sectioned. The inferior pulmonary ligament is divided up to the level of the inferior vein to facilitate the expansion of lower lobe. The main steps of the procedure are summarized in Figure 3.

Left lower lobectomy (LLL)
After sectioning the posterior part of the oblique fissure, the apical segmental artery (A6) and the basal trunk artery (A7 + A8 + A9 + A10) are isolated and either sequentially ligated manually or cut with an endostapler, as convenient. The pulmonary ligament is resected up to the level of the inferior pulmonary vein. The margin of the vein is identified and the vein is isolated and mechanically sectioned. Then, the anterior part of the oblique fissure is sectioned, the left lower bronchus is exposed and mechanically sectioned. The main steps of the procedure were edited in Figure 4.

Right upper lobectomy (RUL)
After sectioning the posterior part of the oblique fissure, the right upper bronchus is isolated and mechanically sectioned. During this manoeuver, care should be taken not to injury the trunk of pulmonary artery taking the dissector close to the posterior wall of the upper bronchus. Following division of the bronchus, the posterior (A2), the anterior (A3) and the apical (A1) segmental pulmonary arteries are isolated and either sequentially ligated manually or cut with an endostapler, as convenient. The lung is retracted posteriorly facilitating dissection of the superior vein that is exposed and mechanically sectioned. During this maneuver, care should be taken not to injury the middle lobe veins. Finally, the horizontal fissure is sectioned with stapler. The inferior pulmonary ligament is divided to help expansion of the right lower lobe. The main steps of the procedure were edited in Figure 5.

Right lower lobectomy (RLL)
After sectioning the posterior part of the oblique fissure, pulmonary artery is identified and the apical segmental artery (A6) and the basal trunk artery (A7 + A8 + A9 + A10) are dissected and either sequentially ligated manually or cut with an endostapler, as convenient. The lower lobe is mobilized by sectioning the inferior pulmonary ligament up to the level of the inferior pulmonary vein. The vein is isolated and mechanically sectioned. The right lower bronchus is then isolated and mechanically resected. During this maneuver, care should be taken not to injury the middle lobe bronchus. Finally, the anterior portion of the oblique fissure is sectioned with a stapler. The main steps of the procedure are summarized in Figure 6.

Lobectomies in case of incomplete fissures
In some patients the fissures between the upper and lower lobes are incomplete or completely fused.
On the right side, the dissection starts at the confluence between the major and minor fissures until the pulmonary artery and its branches are identified. The dissection is carried out between the posterior ascending segmental artery and the superior segmental artery. The posterior hilar dissection is carried out between the inferior margin of the right upper bronchus and the bronchus intermedius. After the space between the right upper bronchus and the intermediate bronchus is developed, a clamp is passed in this space emerging at confluence between the fissures. An endostapler is passed into this space and the posterior superior portion of the right major fissure is then divided. The lobectomy is continued as above reported.
On the left side, the dissection starts in the mid-portion of the oblique fissure, until the pulmonary artery and its branches are identified in their interlobar position. The posterior hilar dissection of the pulmonary artery allows to create a plane between the posterior branches to the upper lobe and the superior segmental branch to the lower lobe. A clamp is then passed in this space emerging at the mid-portion of the oblique fissure. An endostapler is then passed into this place and the posterior superior portion of the oblique fissure is divided. The lobectomy is continued as above reported. Figure 7 shows a RUL in case of completely fused posterior aspect of the major fissure.

Once the lobe is completely resected, it is placed in a specimen bag to avoid chest wall contamination of tumor cells and then retrieved through the same access. A radical mediastinal lymph nodes dissection is performed in a standard fashion. A single 28 Fr drainage is left in the pleural cavity through the same operative incision. Patients are extubated in the operating room and transferred to the surgical ward. The postoperative analgesia is performed with intravenous morphine administered through Patient Controlled-Analgesia (Automed 3300, AceMedical Co., Seoul, Korea) delivery. Morphine 1 mg was given for each request and continuous infusion was at a rate of 1 mg/h. Patients had a 10 min lockout period and a safe higher limit of 20 mg in 4 hours. If Visual Analogue Scale scores exceeded 4/10 scores, rescue analgesia was intravenously administered according to a standardized institutional protocol for pain treatment until the pain was relieved to a level falling below a VAS score <4. PCA was continued for up to 2 days, until patients could tolerate oral opioid medications and/or anti-inflammatory analgesics.
Statistical analysis
We used the Kolmogorov-Smirnov test and graphic histograms to check the normality/skewness of continuous variables data before further analysis, and appropriate statistical tests have been accordingly chosen. Data were summarized as mean and standard deviation (SD) for continuous variables or absolute number and percentage for categorical variables. The comparison of continuous variables was performed with Student t-test or with ANOVA test in case of repeated measures. A P value less than 0.05 was considered statistically significant. MedCalc statistical software (Version 12.3, Broekstraat 52; 9030 Mariakerke; Belgium) was used for the analysis.
Results
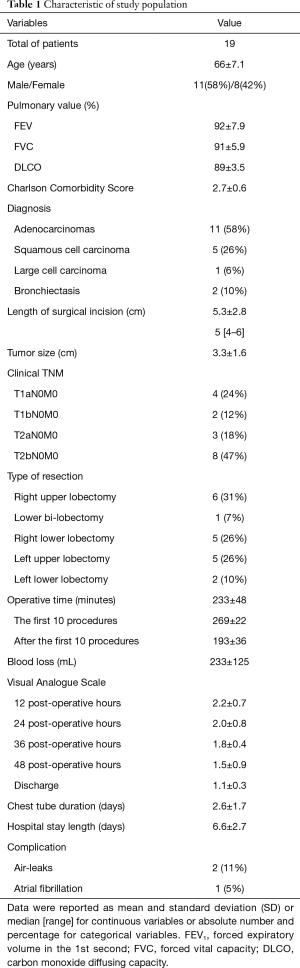
Lobectomy was successfully performed in 19 patients (RUL =6; RLL =5; LUL =6; LLL =2) for lung cancer (adenocarcinoma =11; squamous carcinoma =5; large cell carcinoma =1) or benign disease (bronchiectasis =2). The demographic, clinical and pathological characteristics of our study population are summarized in Table 1. The mean tumor size and length of surgical incision was 3.3±1.6 and 5.3±2.8 cm, respectively.
Full table
The mean operative time was 233±48 minutes, but a significant reduction was observed with the increasing number of the procedure performed. The operative time registered in the first ten procedures was significantly higher than that observed after (269±22 vs. 193±36; P<0.0001; Student t-test). During the entire post-operative time, the VAS score was under the mean level of 3 and there was no significant difference between the value registered at different time points (P=0.14, ANOVA test). The mean chest drainage duration and length of hospital stay were 2.6±1.7 and 6.6±2.7 days, respectively. No mortality or major complications were found; two patients had prolonged air leaks (>5 days) that spontaneously resolved with chest drainage in suction and another patient presented atrial fibrillation. At time of this paper, all patients were alive without recurrence (mean follow-up: 13±3.7 months).
Discussion
The first report of Uniportal VATS was published in 2004 by Rocco et al. (11) for intermediate anatomical procedure as wedge resection of small nodule. Five years later, Gonzalez-Rivas et al. (12) described the first anatomical lung resection as lobectomy using uniportal VATS approach. After that, most complex procedures as bronchial and vascular sleeve resections have been reported and currently there is no-doubt that every thoracic procedure could be carried out with this technique in expert hands (13-16). The development of dedicated surgical instruments, the improvements in optics, and easy access to surgical videos and expert courses contributed to a more rapid expansion of Uniportal VATS that that observed for multi incisions VATS. Despite the benefits of uniportal versus multi incisions VATS is not fully demonstrated, it does seem reasonable that the use of only one incision may result in less pain than multi incisions (17).
Uniportal VATS is an evolution of multi ports VATS, initially described by thoracic surgeons using anterior (Copenhagen technique) or posterior approach (Edinburgh technique) (18). However, anterior approach has more rapidly expanded than posterior and currently is adopted and also modified by the most of thoracic surgeons as the strategy of choice also for uniportal VATS probably because they have found easier and safer to view and control vascular hilar structures first (1-4,17). Despite all, the posterior approach popularized by Richards et al. (18) allows to have a better view of the posterior hilum than anterior approach and it could facilitate the dissection of the bronchi and branches of the pulmonary arteries. Thus, we tried to reproduce the Edinburgh posterior technique using a single incision rather than multi incisions, a technique not largely reported in literature. The surgical incision performed in the triangle auscultatory rather than in the posterior axillar line and the surgeon placed posteriorly rather than anteriorly to patient are the main differences of our technique versus standard anterior uniportal VATS. In line with the geometric configuration of the standard uniportal anterior VATS approach, also in our technique the instruments are used parallel to the optics and directed into the hilar structures through the uniportal incision but the order of dissection is from posterior to anterior, by opening up the fissure first to identify and isolate pulmonary artery branches. Thus, the surgeon has an operative view similar to that of posterolateral thoracotomy and he can easily replicate the same maneuvers performed in open approach.
Our technique adds to the previous advantages of the posterior approach reported by Richards et al. (18) the additional potential advantages as follows: (I) to view the lung from a posterior incision similar to standard posterolateral thoracotomy could facilitate the learning curve and the rapid transition into the uniportal VATS also for surgeons who use a posterolateral thoracotomy and are not familiar with VATS; (II) complex procedure as upper sleeve resection (due to a better view of the bronchus), and (III) an emergency conversion to posterolateral thoracotomy (since the surgeon is yet placed behind the patient) could be easier with posterior over anterior VATS. Obviously, all these advantages are speculative and should be confirmed by future experiences considering that no sleeve resection or conversion is included in our series.
Despite our mean operative time is longer than that of standard lobectomy (4 vs. 2 hours), we observed a decreasing operative time during our ongoing experience that resulted to be about 2 hours for the last procedures. In the same cases the choice of performing a manual ligation of the vessels could additionally explain our long operative time. The mean length of the incision is 4.5 cm in line with that of the standard anterior VATS (ranging from 4–6 cm depending of the lobe to be removed and the size of the tumor) (16). The mean length of hospital stay is 5 days and certainly longer than expected for a minimally invasive procedure as uniportal VATS lobectomy. However, the mean chest drainage duration was 37 hours, in line with other experiences of uniportal anterior VATS lobectomy, and patients were discharged a couple of days later but no during weekend to avoid hospital readmission. That could explain our longer hospitalization.
Our method could present several limitations as follows: (I) the position of the camera in the distal end of the incision can obstruct the insertion and the movement of the stapler; (II) to dissect the bronchus first could increase the risk of injuring the upper branches of pulmonary artery especially during upper right lobectomy; (III) to dissect the fissure first could increase the risk of air-leaks compared to fissure less technique where the dissection of the fissure is the last step of the lobectomy. In our study population, only two patients had air-leaks that spontaneously stopped with chest drainage under suction 7 and 8 days later. Both patients were affected by chronic obstructive pulmonary disease; (IV) the posterior intercostal space is narrower than anterior, thus the extraction of large tumor could be more difficult and increase the post-operative pain. However, in our study population the VAS score was under the mean value of 3 and no difference was found during the different time points.
In conclusion, we proposed an additional strategy for performing uniportal VATS using a posterior approach. Obviously, the feasibility and the reproducibility of this procedure and its potential advantages should be determined by further studies with large patients and long follow-up.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5. [Crossref] [PubMed]
- Rocco G, Martucci N, La Manna C, et al. Ten-year experience on 644 patients undergoing single-port (uniportal) video-assisted thoracoscopic surgery. Ann Thorac Surg 2013;96:434-8. [Crossref] [PubMed]
- Kara HV, Balderson SS, D'Amico TA. Modified uniportal video-assisted thoracoscopic lobectomy: Duke approach. Ann Thorac Surg 2014;98:2239-41. [Crossref] [PubMed]
- Fiorelli A, Vicidomini G, Laperuta P, et al. Pre-emptive local analgesia in video-assisted thoracic surgery sympathectomy. Eur J Cardiothorac Surg 2010;37:588-93. [Crossref] [PubMed]
- Caronia FP, Arrigo E, Fiorelli A. Left upper lobectomy: section of upper pulmonary arteries, upper pulmonary vein and upper bronchus. Asvide 2017;4:464. Available online: http://www.asvide.com/articles/1781
- Caronia FP, Arrigo E, Fiorelli A. Left lower lobectomy: section of lower pulmonary arteries, lower pulmonary vein and lower bronchus. Asvide 2017;4:465. Available online: http://www.asvide.com/articles/1782
- Caronia FP, Arrigo E, Fiorelli A. Right upper lobectomy: section of upper bronchus, upper pulmonary arteries, and upper pulmonary vein. Asvide 2017;4:467. Available online: http://www.asvide.com/articles/1783
- Caronia FP, Arrigo E, Fiorelli A. Right lower lobectomy: section of lower pulmonary artery, lower pulmonary vein and lower bronchus. Asvide 2017;4:468. Available online: http://www.asvide.com/articles/1784
- Caronia FP, Arrigo E, Fiorelli A. Right upper lobectomy in case of incomplete fissure between the upper lobe and lower lobe. Asvide 2017;4:469. Available online: http://www.asvide.com/articles/1785
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, de la Torre M, Fernandez R, et al. Single-port video-assisted thoracoscopic left upper lobectomy. Interact Cardiovasc Thorac Surg 2011;13:539-41. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Uniportal video-assisted thoracoscopic sleeve lobectomy and other complex resections. J Thorac Dis 2014;6:S674-81. [PubMed]
- Caronia FP, Fiorelli A, Santini M, et al. Uniportal bilateral video-assisted thoracoscopic extended thymectomy for myasthenia gravis: A case report. J Thorac Cardiovasc Surg 2015;150:e1-3. [Crossref] [PubMed]
- Caronia FP, Fiorelli A, Santini M, et al. Uniportal Video-Assisted Thoracoscopic Surgery Resection of a Giant Midesophageal Diverticulum. Ann Thorac Surg 2017;103:e365-e367. [Crossref] [PubMed]
- Caronia FP, Fiorelli A, Ruffini E, et al. A comparative analysis of Pancoast tumour resection performed via video-assisted thoracic surgery versus standard open approaches. Interact Cardiovasc Thorac Surg 2014;19:426-35. [Crossref] [PubMed]
- Martin-Ucar AE, Socci L. Why perform uniportal video-assisted thoracic surgery?—multiple considerations. J Vis Surg 2016;2:108. [Crossref]
- Richards JM, Dunning J, Oparka J, et al. Video-assisted thoracoscopic lobectomy: the Edinburgh posterior approach. Ann Cardiothorac Surg 2012;1:61-9. [PubMed]


