Robot-assisted surgery for posterior mediastinal mass
In the last decade, robotic surgery is increasingly becoming an essential instrument in the hands of thoracic surgeons. Indeed, the Robotic Surgical Systems (da Vinci, Intuitive Surgical, Inc., Sunnyvale, CA, USA), particularly the latest models, the SI system and the latest XI system, are used to perform lung resection and exeresis of mediastinal lesions (1-3). Thanks to their features it is possible to work in a comfortable and secure manner in narrow spaces, such as the anterior mediastinum, or in remote areas, such as the posterior mediastinum or the costal-phrenic areas. Robotic surgery allows a mini-invasive approach overcoming the limits that characterize video-assisted thoracic surgery (e.g., complex maneuverability of the instruments in close or deep spaces, 2-dimensional and limited vision). As a matter of fact, surgical procedures are easier thanks to the 3D magnified vision, the surgeon’s direct control of the camera, the possibility to have instruments with a large range of articulation and movements, the filtration of the physiological tremor of the hands (4,5).
Currently, the use of robotic surgery to remove mediastinal lesions has become a routine choice, guaranteeing excellent results. Several Authors have described the robotic surgical technique and its results for the treatment of anterior mediastinal lesions, in particular of the thymic gland disease (6-8). However, only few authors have reported their experience on the application of robotic system for posterior mediastinal tumors (9,10).
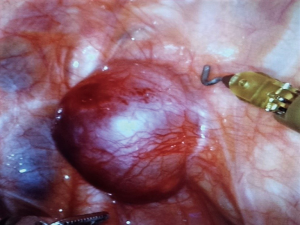
Neurogenic tumors are the most common type of posterior mediastinal lesions. In most cases, the patients are asymptomatic and the diagnosis is accidental. Usually the neoplasm is benign, well defined, localized in a paravertebral area, arising from peripheral nerves, as intercostal nerve, or sympathetic nerves (Figure 1). Other lesions located in the posterior mediastinum can be cysts, esophageal tumors, lymphadenopathy, infectious or inflammatory lesions (11).
Despite the uncomfortable site, the removal of the masses localized in the posterior mediastinum using robotic technique is usually described as a simple and safe procedure. For these characteristics this kind of procedure could therefore represent the first step of the learning curve for the surgeon starting a thoracic robotic program (12).
The authors described different port mapping (9,12-15). The exeresis of posterior mediastinal lesions consists of three or four centimetric surgical ports. Guo et al. illustrated an approach with three surgical accesses: camera port at 5th intercostal space at mid-axillary line, the posterior port in 8th intercostal space, at the midpoint between posterior axillary line and subscapular line, and the anterior port at 3th intercostal space between anterior axillary line and midclavicular line (16).
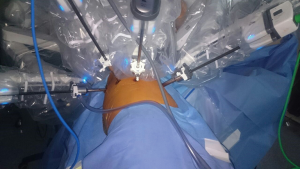
To obtain a standardization of technique, a useful port mapping could be the port mapping used also for lung resection. After the intubation, the patient must be positioned in lateral decubitus, with operating table flexed at the level of the inferior border of the scapula. The positioning is mandatory in order to obtain the alignment of the scapula and the hip, preventing potential injuries to the hip and the camera port.
The camera port is positioned in the 7th or 8th intercostal space on the posterior axillary line using a 30° camera. When possible, given the variability the chest wall, the posterior ports are positioned in the same intercostal space, each of them 6 cm from the camera port with a second optional port positioned in the auscultatory triangle. The anterior port is positioned over the diaphragm, in the 5th–6th intercostal space on the anterior axillary line (Figure 2). It is always highly recommended to verify the internal position of each surgical port with the camera, in order to ensure an adequate distance between the arms. The CO2 insufflation (5–8 mmHg), can be useful to increase the space available for maneuverability thanks to the collapsing of the lung and the flection diaphragm (17).
The fourth arms already reported, is not strictly indispensable, although t it can be applied to use a grasper to retract the lung achieving a better vision. From a technical point of view, the use of all four arms of the robotic system is recommended as it represent a good exercise for the surgeon at the beginning of the robotic experience.
The used instruments can be the monopolar (e.g., Hook or Spatula, Intuitive Surgical) or the bipolar instruments (e.g., Maryland or Fenestrated Bipolar, Intuitive Surgical), as reported by Guo et al., and if used in the fourth arm a grasper (e.g., Cadiere, Prograsp, Intuitive Surgical) (16).
Few authors reported their experience, usually concerning a small series about the removal of masses located in posterior mediastinum using robotic surgical system.
The robotic system allows the execution of the surgical procedure with exceptional precision and safety, guaranteeing minimization of surgical trauma and surgical manipulation of the mass. Therefore, robotic surgery, is characterized by less pain, less hospital-stay, fewer complications, good cosmetic results and quick return to daily activities (18).
The use of robotic surgical system for surgery of posterior mediastinal masses to be a safe and comfortable mini-invasive technique, representing a useful instrument for the treatment of lesions located in narrow spaces, generally barely reachable.
Acknowledgements
The Authors thank Teresa Hung Key for assistance with this article.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wei B, Cerfolio RJ. Robotic Lobectomy and Segmentectomy: Technical Details and Results. Surg Clin North Am 2017;97:771-82. [Crossref] [PubMed]
- Yamashita S, Yoshida Y, Iwasaki A. Robotic Surgery for Thoracic Disease. Ann Thorac Cardiovasc Surg 2016;22:1-5. [Crossref] [PubMed]
- Melfi FM, Fanucchi O, Mussi A. Minimally invasive mediastinal surgery. Ann Cardiothorac Surg 2016;5:10-7. [PubMed]
- Liang H, Liang W, Zhao L, et al. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Fok M, Bashir M, Harky A, et al. Video-Assisted Thoracoscopic Versus Robotic-Assisted Thoracoscopic Thymectomy: Systematic Review and Meta-analysis. Innovations (Phila) 2017;12:259-64. [Crossref] [PubMed]
- Gkouma A. Robotically assisted thymectomy: a review of the literature. J Robot Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Rueckert J, Swierzy M, Badakhshi H, et al. Robotic-assisted thymectomy: surgical procedure and results. Thorac Cardiovasc Surg 2015;63:194-200. [Crossref] [PubMed]
- Karagkounis G, Uzun DD, Mason DP, et al. Robotic surgery for primary hyperparathyroidism. Surg Endosc 2014;28:2702-7. [Crossref] [PubMed]
- Xu S, Liu B, Wang X, et al. Robotic thoracic surgery of the posterior superior mediastinal mass. Ann Transl Med 2015;3:127. [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Operative techniques in robotic thoracic surgery for inferior or posterior mediastinal pathology. J Thorac Cardiovasc Surg 2012;143:1138-43. [Crossref] [PubMed]
- Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest 2005;128:2893-909. [Crossref] [PubMed]
- Balduyck B, Hendriks JM, Lauwers P, et al. Quality of life after anterior mediastinal mass resection: a prospective study comparing open with robotic-assisted thoracoscopic resection. Eur J Cardiothorac Surg 2011;39:543-8. [Crossref] [PubMed]
- Perez-Cruet MJ, Welsh RJ, Hussain NS, et al. Use of the da Vinci minimally invasive robotic system for resection of a complicated paraspinal schwannoma with thoracic extension: case report. Neurosurgery 2012;71:209-14. [PubMed]
- Pacchiarotti G, Wang MY, Kolcun JP, et al. Robotic paravertebral schwannoma resection at extreme locations of the thoracic cavity. Neurosurg Focus 2017;42:E17. [Crossref] [PubMed]
- Ruurda JP, Hanlo PW, Hennipman A, et al. Robot-assisted thoracoscopic resection of a benign mediastinal neurogenic tumor: technical note. Neurosurgery 2003;52:462-4; discussion 464. [Crossref] [PubMed]
- Guo W, Yang S, Jin R, et al. Robot-assisted surgery for posterior superior mediastinal mass. AME Med J 2017;2:10. [Crossref]
- Kocher GJ, Schmid RA, Melfi FM. Robotic lobectomy: tips, pitfalls and troubleshooting. Eur J Cardiothorac Surg 2014;46:e136-8. [Crossref] [PubMed]
- Kamel MK, Rahouma M, Stiles BM, et al. Robotic Thymectomy: Learning Curve and Associated Perioperative Outcomes. J Laparoendosc Adv Surg Tech A 2017;27:685-90. [Crossref] [PubMed]