Risk factors of pre-operational aortic rupture in acute and subacute Stanford type A aortic dissection patients
Introduction
Acute and subacute Stanford type A aortic dissection (ATAAD), a vascular emergency characterized by abrupt onset and catastrophic outcome, remains a major challenge for both cardiovascular surgeons and hospitals, although the reported incidence of ATAAD is low (3.5 to 6/100,000) (1,2). Without timely surgical repair, ATAAD can quick lead to fatal conditions including aortic rupture and multi-organ malperfusion. Despite current advances in ATAAD diagnosis and treatment, the early mortality rate remained at 25.4% and the 1-year mortality at 37.4% in the United States in 2011 (3). Previous research has suggested some pre- and peri-operative factors associated with ATAAD mortality, including preoperative ST-elevation, older age, abrupt onset of chest pain, hypotension, pulse deficit, as well as comatose state (4-6). More recent reports also noted that biochemical parameters, including levels of D-dimer, white blood cell count, N-terminal pro-brain natriuretic peptide, and serum lactic acid level, were associated with ATAAD mortality (7-10).
Among all causes of mortality in cases of ATAAD, aortic rupture is the most dangerous one, accounting for one-third of deaths reported in patients treated conservatively without surgery (6). Afifi et al. recently reported not only a lower short-term survival rate, but also a lower long-term survival rate in ATAAD patients who experienced frank rupture (11). However, current research has provided scarce data on the potential factors associated with the risk of rupture in ATAAD. Therefore, this study aimed to investigate the potential risk factors associated with aortic rupture in ATAAD patients.
Methods
Patients
Between May 2013 and May 2016, a total of 329 patients diagnosed with ATAAD in the Department of Cardiothoracic Surgery of Changhai Hospital, Shanghai were recruited for this study. A retrospective review of their medical records was performed. This study was approved by the Committee on Ethics of Biomedicine Research, Second Military Medical University, Shanghai (No. SMMUEC2015-46).
Data collection
A standardized form was used to collect clinical information. Data included patient demographics, history of disease, type of dissection according to the Stanford classification, time of onset, admission date, operation parameters, and cause of death was recorded. Other peri-operational parameters including blood pressure, heart rate, and biochemical test results were also recorded.
Definitions and measurements
In this study, all patients were diagnosed by computed tomography angiography (CTA) examination of the aorta. The Stanford type A aortic dissection was defined when the dissection involved the ascending aorta (12). The acute and subacute phrases were defined if the time from the onset of the symptoms to admission in our center was less than 14 days. According to the medical record, aortic rupture in ATAAD was defined if these clinical manifestations were demonstrated: sudden loss of consciousness, autonomous cessation of breathing, loss of pulse, and gradual stopping of heartbeat. Blood samples were obtained within 2 hours after admission; otherwise, the tested values were defined as missed. The pain assessment on admission was scored according to a range from 0 to 10, where ‘0’ meant painless and ‘10’ meant the most severe pain. In this study, we graded pain in four categories: ‘0’ meant painless (matching the 0 score in the medical record), ‘1’ meant slight pain (matching 1 or 2 score, analgesic was not required), ‘2’ meant pain must be managed (matching 3–10), and ‘3’ meant recurrent pain within 3 hours after pethidine intramuscular injection with dosing according to body weight (1 mg/kg). Shock was defined by a record of systolic blood pressure less than 80 mmHg after the onset of symptoms. Respiratory failure was defined by the requirement and use of ventilator support. D-dimer levels were measured using a STA-Evolition (Diagnostica Stago, France). Troponin levels was measured using a Beckman Access II (Beckman Coulter, USA).
Statistical methods
Statistical analyses were performed with SAS software (version 9.3, SAS Institute, Inc., Cary, NC, USA). Continuous variables were presented as mean ± standard deviation (SD) or median and interquartile ranges, dependent on distribution normality. Missing values were replaced by either mean or median value of the variables dependent on their distribution. Comparison of continuous variables between two groups was conducted by Student’s t-test when variables were normally distributed. When variables were not distributed normally, Wilcoxon rank sum test was used. Categorical variables were presented as proportions and analyzed with K. Pearson chi-square tests or Fisher-Irwin test. To identify risk factors associated with the risk of rupture, significant variables were first detected by simple logistic regression analysis and further analyzed in stepwise multivariable logistic regression. Odds ratio (OR) and 95% confidence interval (95% CI) were calculated to assess the association. All P values were two-side, and statistical significance was considered when P was less than 0.05.
Results
Patient characteristics
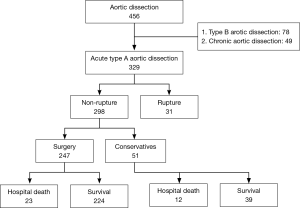
Among the total of 329 patients with ATAAD (Figure 1), 31 patients experienced aortic rupture and died. In the non-ruptured group (n=298), 247 patients underwent surgery, and 51 patients underwent conservative treatment due to economic reasons or multi-organ dysfunction. A total of 66 patients died in the hospital due to ATAAD, and the overall in-hospital mortality of ATAAD was 20.0%. Rupture accounted for 47% of the cases of death in the hospital. The patients in the ruptured group were significantly older (60.2±13.1 vs. 50.1±12.8 years; P<0.001; Table 1). There were no significant differences in the gender proportion, time from onset to surgery/death, or history of disease between the ruptured and non-ruptured groups. Among the 31 patients with rupture, 6 (19.4%) patients developed rapture within 3 hours on admission, 8 (25.8%) patients experienced rupture during the midnight waiting for surgery on the coming day, 9 (29.0%) patients refused surgery, and 8 (25.8%) patients had severe complications including coma and multi-organ failure such as respiratory, liver and kidney failure, abdominal pain and bloody stool. Therefore, conservative treatments were chosen.
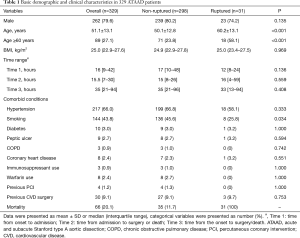
Full table
Clinical presentation of ATAAD upon admission
The details of the clinical presentation of ATAAD patients upon admission to the hospital are presented in Table 2. The ruptured group had a lower systolic blood pressure than the non-ruptured group (135.1±23.0 vs. 122.3±32.4 mmHg, P=0.040). Pain assessment was also compared between the two groups. Overall, anodyne was required in 80 (24.3%) ATAAD patients, and a significantly higher proportion of patients in the ruptured group (19/31, 61.3%) than in the non-ruptured group (61/298, 20.5%; P<0.001; Table 2) required anodyne. There were also fewer patients with aortic regurgitation by ultrasonic cardiography in the ruptured group (46.3% vs. 16.1%, P=0.001). Compared to the non-ruptured group, the ruptured group had higher incidences of shock (22.6% vs. 3.7%; P=0.001), coma (16.1% vs. 1.0%; P<0.001), and deficit limb pulse (18.1%% vs. 41.9%; P=0.002).
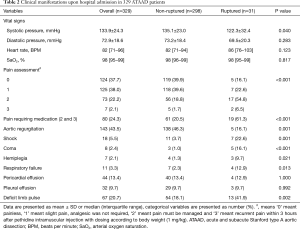
Full table
Biochemical results
The differences in the biochemical results are presented in Table 3. In the arterial blood gas analysis, oxygen pressure was significantly higher in the non-ruptured group [90 mmHg (74–132) mmHg] than in the ruptured group [71 mmHg (64–101) mmHg], P=0.006]. As expected, more patients had hypoxemia in the rupture group (74.2% vs. 37.6%, P<0.001). The rupture group also had significantly higher lactate levels compared to the non-ruptured group (P=0.016). More importantly, the platelet and blood coagulability test results demonstrated that the rupture group had a significantly lower platelet count (P=0.012), longer prothrombin time (P<0.001), and higher D-dimer level [ruptured: 16.00 (9.44–16.00) µg/mL vs. non-ruptured: 6.12 (2.72–16.00) µg/mL; P=0.003]. Kidney and liver function tests also showed that the ruptured group had higher serum urea nitrogen, creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and troponin levels, indicating more severe systematic multi-organ injury. No differences were found in the white blood cell count or total bilirubin, creatine kinase-MB (CK-MB), or C-reactive protein (CRP) levels between the ruptured and non-ruptured groups.
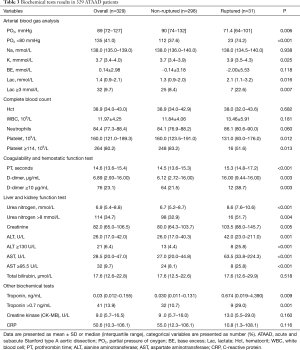
Full table
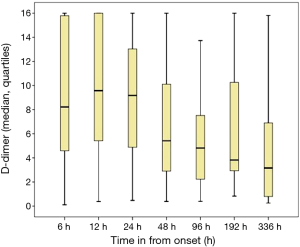
The dose-time distribution curves for the D-dimer of the 329 patients are also presented (Figure 2). The D-dimer level reached a peak level in 8–12 hours after onset and then gradually decreased over the next 14 days.
Multivariate analysis for predictors of ATAAD in-hospital rupture
Multivariable analysis on all of these significantly different factors using stepwise selection was used to identify significant variables associated with aortic rupture in ATAAD patients (Table 4). Four independent risk factors were identified: shock (OR: 8.12; 95% CI, 1.10–59.85, P=0.040), pain requiring medication (OR: 12.67; 95% CI, 2.43–66.09; P=0.003), a troponin level >0.7 ng/mL (OR: 9.28; 95% CI, 1.72–50.06; P=0.010), and a D-dimer level ≥10 µg/mL (OR: 13.37; 95% CI, 2.18–81.97; P=0.005).
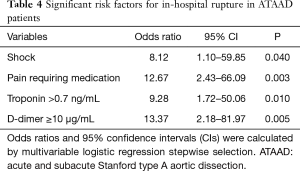
Full table
Discussion
This study described the clinical characteristics of acute and subacute type A aortic dissection and proposed the potential risk factors for aortic rupture. The in-hospital aortic rupture rate was 9.4%, and the rupture accounted for 47% of in-hospital deaths among ATAAD patients. The patients who experienced rupture were older, had lower systolic pressure, had more painful manifestation, had more systematic complications, and had worse blood coagulability. We propose that a presentation of shock, pain requiring medication, and higher levels of troponin and D-dimer is independently associated with rupture in cases of ATAAD.
In this study, the in-hospital mortality rate of ATAAD (20.0%) was comparable to other previous studies (6,13), and the mortality rate of the patients treated without surgery, including that caused by rupture, was also comparable to a previous report in conservatively treated patients (52.4% vs. 56.2%) (6). The rupture rate (9.4%), however, was much lower than that in the study by Afifi et al. 2016 (15.3%) (11), which may due to the different time ranges of patient recruitment as Afifi et al. recruited patients much earlier than us. Afifi et al. also demonstrated risk factors related to rupture in cases of ATAAD including older age, female gender, dilated aorta, hypotension, and cardiac arrest (11). However, we demonstrated different risk factors associated with the rupture risk.
In this study, the patients who presented with shock had an 8.12-fold greater risk of rupture than patients without shock (P=0.040), which is in accordance with previous reported relations between shock and ATAAD mortality. In the study by Bossone et al. with the data of the International Registry of Acute Aortic Dissection (14), 407 (15.1%) of the 2704 ATAAD patients presented with shock upon admission, and the in-hospital mortality was significantly higher in these patients with shock than patients without shock (30.2% vs. 23.9%, P=0.007). More importantly, shock at presentation was reported to be an independent risk factor for surgical mortality (15,16) and long-term mortality (17). These data indicate that an early intervention in ATAAD patients who presented with shock may help reduce the rupture and mortality rates. Ischemia and hypoxia caused by shock may lead to vulnerability of the aortic wall, which in turn leads to aortic rupture.
Pain is the most common symptom of acute aortic dissection. In our study, pain assessment was graded into four categories, and 63% patients presented with overt pain. This proportion was lower than in previous studies (14,15). It was also demonstrated that the abrupt pain is associated with higher in-hospital mortality (6). The pain in ATAAD is caused by compression and stretching of the para-aortic nerve plexus and peripheral nerves. Therefore, pain is directly related to tension of the aortic lesion. A consistent severe pain may suggest continuous high tension in the aortic wall. Moreover, blood pressure and heart beat are usually not easily controlled in patients experiencing severe pain. Our study demonstrated that a significantly higher proportion of patients in the ruptured group required analgesic. More importantly, medication-requiring pain was demonstrated to be an independent risk factor for aortic rupture.
Our study also indicates the potential role of myocardial injury likely caused by coronary malperfusion in the progression of ATAAD. As a complication of ATAAD, coronary malperfusion can be demonstrated by elevated serum markers and abnormal electrocardiogram or echocardiography results as in previous studies (16,18). In a study with 502 ATAAD patients, coronary malperfusion was an independent risk factor for in-hospital mortality (16). In another study based on the data of the German Registry for Acute Aortic Dissection Type A registry, pre-operative coronary malperfusion was an independent predictor for all post-operative organ malperfusion and early mortality (4). In our study, a serum troponin level >0.7 ng/mL was significantly associated with a 9.28-times higher risk for ruptured ATAAD. Therefore, the elevation of serum troponin suggests the severity of aortic dissection and potential unstable systemic circulation resulting from acute myocardial injury, which is associated with aortic rupture.
Our data also confirm the important role of D-dimer in the prognosis of ATAAD. Besides previous evidence on D-dimer as a biological marker for diagnosing acute aortic dissection (19,20) and predicting in-hospital mortality (21-23), scarce data have been published on its relationship with rupture. In the present study, we have demonstrated that a D-dimer ≥10 µg/mL was an independent risk factor of aortic rupture. The underlying mechanism remains unclear. Weber et al. suggested that the D-dimer level was significantly associated with the severity of aortic dissection and it was much higher in patients with ATAAD than in those with type B aortic dissection (24). Previous research also demonstrated a two-way interaction between inflammation and the coagulation system (25). Therefore, pre-operational aortic rupture could be due to multiple factors. In the group of patients without aortic rupture, we also demonstrated an increase trend in D-dimer levels during the first 12 hours followed by a gradual decrease until day 14 (Figure 2). These results were different from those of a previous study by Suzuki et al. (26), which included 87 ATAAD patients. They found that D-dimer levels decreased after 6 hours from the onset (26). Therefore, the role and trend of D-dimer changes in ATAAD require further investigation, and our data suggest its potential value in association with aortic rupture in cases of ATAAD.
This study also has several limitations. As a single-centered retrospective analysis, this study had inherent limitations and biases, including the relatively fewer cases in the ruptured group, missing data on biochemical results and CTA, and the lack of prior treatment records. Moreover, most of the patients in this study were treated with surgery within a very short time after admission, and previous co-morbidity data and echocardiographic data were incomplete.
In conclusion, aortic rupture is a catastrophic complication in Stanford acute type A aortic dissection and it accounts for 47% of total in-hospital mortality among these patients. Presentation with shock, medication-requiring pain, serum troponin >0.7 ng/mL, and D-dimer ≥10 µg/mL were independent risk factors for aortic rupture in ATAAD patients. More data from other centers are needed to confirm our results, and further clinical and laboratory research is warranted to elucidate the role of these risk factors in the prognosis of ATAAD.
Acknowledgements
Funding: This work was sponsored by Health System Foundation of Shanghai (2014ZYJB0401, ZY Xu) and Natural Science Foundation of Shanghai (16ZR1400900).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Committee on Ethics of Biomedicine Research, Second Military Medical University, Shanghai (No. SMMUEC2015-46).
References
- Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013;127:2031-7. [Crossref] [PubMed]
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004;79:176-80. [Crossref] [PubMed]
- Mody PS, Wang Y, Geirsson A, et al. Trends in aortic dissection hospitalizations, interventions, and outcomes among medicare beneficiaries in the United States, 2000-2011. Circ Cardiovasc Qual Outcomes 2014;7:920-8. [Crossref] [PubMed]
- Czerny M, Schoenhoff F, Etz C, et al. The impact of pre-operative malperfusion on outcome in acute type a aortic dissection: results from the GERAADA Registry. J Am Coll Cardiol 2015;65:2628-35. [Crossref] [PubMed]
- Kawahito K, Adachi H, Yamaguchi A, et al. Preoperative risk factors for hospital mortality in acute type A aortic dissection. Ann Thorac Surg 2001;71:1239-43. [Crossref] [PubMed]
- Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type a aortic dissection. Circulation 2002;105:200-6. [Crossref] [PubMed]
- Zhang R, Chen S, Zhang H, et al. Biomarkers investigation for in-hospital death in patients with Stanford type a acute aortic dissection. Int Heart J 2016;57:622-6. [Crossref] [PubMed]
- Yoshimuta T, Yokoyama H, Okajima T, et al. Impact of elevated D-Dimer on diagnosis of acute aortic dissection with isolated neurological symptoms in ischemic stroke. Circ J 2015;79:1841-5. [Crossref] [PubMed]
- Fan X, Huang B, Lu H, et al. Impact of admission white blood cell count on short- and long-term mortality in patients with type A acute aortic dissection: An Observational Study. Medicine (Baltimore) 2015;94:e1761. [Crossref] [PubMed]
- Bennett JM, Wise ES, Hocking KM, et al. Hyperlactemia predicts surgical mortality in patients presenting with acute stanford type-A Aortic Dissection. J Cardiothorac Vasc Anesth 2017;31:54-60. [Crossref] [PubMed]
- Afifi RO, Sandhu HK, Leake SS, et al. Determinants of operative mortality in patients with ruptured acute type A aortic dissection. Ann Thorac Surg 2016;101:64-71. [Crossref] [PubMed]
- Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation 2003;108:628-35. [Crossref] [PubMed]
- Teman NR, Peterson MD, Russo MJ, et al. Outcomes of patients presenting with acute type A aortic dissection in the setting of prior cardiac surgery: an analysis from the International Registry of Acute Aortic Dissection. Circulation 2013;128:S180-5. [Crossref] [PubMed]
- Bossone E, Pyeritz RE, Braverman AC, et al. Shock complicating type A acute aortic dissection: Clinical correlates, management, and outcomes. Am Heart J 2016;176:93-9. [Crossref] [PubMed]
- Rampoldi V, Trimarchi S, Eagle KA, et al. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection score. Ann Thorac Surg 2007;83:55-61. [Crossref] [PubMed]
- Pacini D, Leone A, Belotti LM, et al. Acute type A aortic dissection: significance of multiorgan malperfusion. Eur J Cardiothorac Surg 2013;43:820-6. [Crossref] [PubMed]
- Girdauskas E, Kuntze T, Borger MA, et al. Surgical risk of preoperative malperfusion in acute type A aortic dissection. J Thorac Cardiovasc Surg 2009;138:1363-9. [Crossref] [PubMed]
- Kawahito K, Adachi H, Murata S, et al. Coronary malperfusion due to type A aortic dissection: mechanism and surgical management. Ann Thorac Surg 2003;76:1471-6; discussion 1476. [Crossref] [PubMed]
- Ersel M, Aksay E, Kiyan S, et al. Can D-dimer testing help emergency department physicians to detect acute aortic dissections? Anadolu Kardiyol Derg 2010;10:434-9. [Crossref] [PubMed]
- Nazerian P, Morello F, Vanni S, et al. Combined use of aortic dissection detection risk score and D-dimer in the diagnostic workup of suspected acute aortic dissection. Int J Cardiol 2014;175:78-82. [Crossref] [PubMed]
- Weber T, Rammer M, Auer J, et al. Plasma concentrations of D-dimer predict mortality in acute type A aortic dissection. Heart 2006;92:836-7. [Crossref] [PubMed]
- Tian L, Fan X, Zhu J, et al. Plasma D-dimer and in-hospital mortality in patients with Stanford type A acute aortic dissection. Blood Coagul Fibrinolysis 2014;25:161-6. [Crossref] [PubMed]
- Wen D, Du X, Dong JZ, et al. Value of D-dimer and C reactive protein in predicting inhospital death in acute aortic dissection. Heart 2013;99:1192-7. [Crossref] [PubMed]
- Weber T, Hogler S, Auer J, et al. D-dimer in acute aortic dissection. Chest 2003;123:1375-8. [Crossref] [PubMed]
- Levi M, van der Poll T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc Med 2005;15:254-9. [Crossref] [PubMed]
- Suzuki T, Distante A, Zizza A, et al. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation 2009;119:2702-7. [Crossref] [PubMed]