Atrial septal defect and exercise capacity: value of cardio-pulmonary exercise test in assessment and follow-up
Introduction
Nearly four decades ago, the World Health Organization stated that functional capacity explorations best reflected the impact of a chronic disease on quality of life (1). In chronic heart failure, several studies have demonstrated a correlation between maximal oxygen uptake (VO2max) and both quality of life and prognosis (2-4). Similar results have also been found in patients with congenital heart diseases (CHDs) (5-9) and cardio-pulmonary exercise test (CPET) is now recommended in the follow-up of this population (10).
Atrial septal defects (ASDs) represent the second most frequent CHD, with a worldwide reported birth prevalence of 2.6 per 1,000 live births (11). Undiagnosed ASDs remain significant in paediatrics (12) and their incidence in adulthood reaches the rates of 1 in 5,000–10,000 (13). Before the 90’s, cardiac surgical repair was the only option for ASD; therefore, only large defects with significantly increased pulmonary blood flow (Qp/Qs >2) underwent heart surgery. Nowadays, percutaneous catheter closure is the first line therapy for most patients with ostium secundum ASDs (10). Indications of ASD closure have been progressively extended to smaller defects, ASDs with moderate pulmonary hypertension, elderly patients, and younger children (14).
Exercise capacity of patients with unrepaired ASDs depends on the importance of the shunt, the right ventricular (RV) function and volume overload, the level of pulmonary arterial pressure, and the occurrence of arrhythmias. For repaired ASDs, exercise capacity also depends on the delay before closure and the type of procedure (catheter or surgery) (15).
This review focuses on the value of CPET in assessment and follow-up of patients with repaired and unrepaired ASDs.
Methods
We searched three electronic databases on October 2017 (PubMed, EMBASE, and Web of Science) by using a combination of the terms “atrial septal defect” and “exercise test”. We also used the terms “cardio-pulmonary exercise test” “CPET”, “VO2” “maximum oxygen uptake” “ASD” “peak VO2” “VO2max” in combination. The selection criteria were as follows: CPET assessment at diagnosis, CPET follow-up after ASD device closure or surgical procedure, CPET follow-up of complications (pulmonary hypertension, arrhythmia, and heart failure) and articles written in English. We did not set any restriction to study setting, era, or locale. Paediatric and adult patients were eligible. In title and abstract screening, two reviewers (P.A. and A.G.) independently reviewed articles identified by the search. Studies identified in title or abstract screening were included for full-text review.
Results
We selected 32 original studies and 2 review articles. One article in Polish and one in German were not selected (16,17). Most studies did not detail the maximum exercise test criteria and/or the existence of a plateau of VO2. Therefore we purposely referred to “peak VO2” in this review. The main CPET parameters are reported and explained in Table 1 (18). The value of these parameters in repaired and unrepaired ADS are summarized in Table 2.
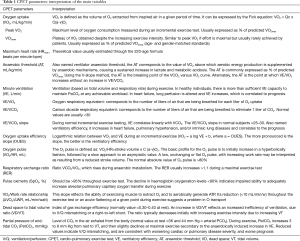
Full table
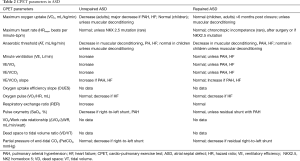
Full table
CPET in unrepaired ASDs
In most reported studies, patients with ASDs have an impaired exercise capacity and their VO2max is reduced until 60% of predicted values and decreases with age (19-24), including minor reduction even for asymptomatic patients (25). Despite the lack of longitudinal cohort CPET studies among ASD patients, it is well established that symptoms usually appear during adulthood and may compromise exercise capacity in older patients (26).
However, the link between peak VO2 and invasive haemodynamic evaluation at rest [mean pulmonary arterial pressure (mPAP), Qp/Qs ratio] remains unclear (27-30). Some studies found a correlation between Qp/Qs and peak VO2 (21,22,29), whereas some others did not (20,25,31).
In ASD patients, RV function may also represent a limiting factor. Indeed, physiologically, the RV mean power is defined as the product of RV cardiac output by mPAP) and is linearly correlated to peak VO2. Van De Bruaene et al. have shown that, compared to healthy controls, the workload of the RV in patients with an open ASD was higher at rest, due to a left-to-right shunt, and at peak exercise, due to an additional increase in mPAP (32). Therefore, during exercise, a higher increase in RV afterload may affect its function, even in asymptomatic ASD without volumetric overload and normal RV function evaluated at rest (33).
Other CPET parameters may be altered in ASDs. The ventilatory efficiency measured by the VE/VCO2 slope increases in ASDs associated with heart failure, RV dysfunction, pulmonary hypertension and/or lung disease (18,20,21,23). The VE/VCO2 slope correlates to prognosis in heart failure (34) and needs to be monitored in the follow-up of CHD patients, as now recommended (10).
The ventilatory anaerobic threshold (AT) may also be impaired in ASDs, as a result of muscular deconditioning, like many patients with simple and complex CHD (8). The “vicious circle” of deconditioning includes dyspnoea at exercise, sedentary lifestyle, overweight and a lack of motivation for sports and exercise. Yet, physical activity and sports are in most cases authorized and even recommended in ASDs (35). Restrictions only concern scuba diving in the presence of a small shunt (risk of paradoxical air emboli), competitive sports in case of symptomatic atrial or ventricular arrhythmias and high intensity sports in case of pulmonary arterial hypertension (PAH) (pulmonary arterial systolic pressure >40 mmHg) (36). Therefore, in the situation of muscular deconditioning diagnosed by CPET, cardiac rehabilitation may be considered for ASD patients (4,37,38).
CPET in repaired ASDs
The large majority of reported studies found a significant improvement in exercise capacity after ASD closure, with both surgical and catheter procedures (15,39). The peak VO2 gradually improves after the procedure (30,40-42), and sometimes reaches normal values in the long term (31). Only one study found no improvement of peak VO2, anaerobic threshold and oxygen pulse after ASD catheter closure, but the cohort was small (N=9) and the delay after procedure was limited, between 1 and 7 months (28).
Once the ASD is closed, the reduction of volumetric overload is associated with a rapid RV remodelling (≅1 month after the procedure) and a significant exercise capacity improvement (≅6 months after the procedure) (40,41,43,44). Along with the peak VO2, the anaerobic threshold, the ventilatory parameters and the oxygen pulse also improve after ASD closure (42,45). At exercise, the stress-induced pulmonary hypertension reduces, the RV means power increases and the RV strain improves (32,46,47). The improvement in terms of exercise capacity after ASD closure concerns patients of all ages, even elderly ones (40,43,48), but is more marked when the procedure occurs at an early stage during childhood (49). However, even when ASD closure is performed in childhood, some patients may have RV strain anomalies more than three decades after the procedure (50). Therefore, CPET remains useful in the long-term follow-up for all patients with a history of ASD.
The haemodynamic status before ASD closure stands as a main determinant for the exercise capacity improvement after the procedure. Indeed, exercise capacity in patients with large left-to-right shunt increases after ASD closure regardless of whether they had high pulmonary arterial pressure (30).
Interestingly, even asymptomatic patients and patients with sub-normal peak VO2 before ASD closure seem to improve their exercise capacity after the procedure (22,25). Similarly, some patients with insignificant shunt (Qp/Qs <1.5) may also benefit from ASD closure in terms of peak VO2, oxygen pulse, ventilatory efficiency and quality of life (45). Indeed, a normal haemodynamic status at rest in patients with ASDs might not be predictable of a normal haemodynamic adaptation at exercise.
No randomized trial compared catheter and surgical ASD closures in terms of CPET parameters’ variation. However, the observational study from Suchon et al. reported a higher decrease in VE/VCO2 slope after catheter than after surgical closure, at 1-year follow-up (39). Similarly, Van De Bruaene et al. showed a gap of 18% in terms of ventilatory efficiency between surgically treated patients and healthy controls, more than 7 years after surgery (23). Physiologically, pulmonary hyper perfusion may deteriorate the lung viscoelastic properties, resulting in remodelling of the lung parenchyma and fibrotic changes (51-53). In this context, the age at cardiac surgery appears to be determinant for the lungs’ ability to preserve or regain their compliance (49).
CPET and comorbidities related to ASDs
As discussed in the previous paragraph, heart failure, PAH and arrhythmia are the main complications of repaired or unrepaired ASDs, for which CPET may be useful.
The existence of an impaired exercise capacity after ASD closure may be associated with RV dysfunction and/or abnormal vascular pulmonary response to exercise (54). For instance, patients with a moderate left-to-right shunt (Qp/Qs <3) associated with elevated pulmonary arterial pressure (systolic PAP >50 mmHg) have an impaired peak VO2, which might not improve after ASD closure (30).
In patients with mild tricuspid insufficiency observed after ASD closure, the exercise capacity is more reduced. Mild tricuspid insufficiency occurs more frequently in older patients and in patients with higher mPAP at peak exercise (55). It could be considered as a marker of subclinical persistent diastolic pressure load on the right ventricle, even after ASD closure.
The exercise capacity is impaired in case of RV dysfunction, as a result of early and/or prolonged RV volume overload, but possibly also as sequel of surgery (50).
A right-to-left shunt at exercise will lead to desaturation and decrease in PetCO2. Therefore, CPET may identify patients who would benefit or not from ASD closure, regarding the risk of developing pulmonary arterial vasculopathy (15). Indeed, the deoxygenated venous blood shunting to the systemic circulation causes a disproportionate rise in CO2 levels and a reflex increase in ventilation (18). This condition commonly occurs in PAH with patent foramen ovale (PFO). Patients with PAH associated with ASD have an important decrease of exercise capacity associated with hyperventilation, attested by a VE/VCO2 ratio increase. Indeed, in the most severe physiology, i.e., the Eisenmenger syndrome, the peak VO2 is the lowest and the VE/VCO2 slope the highest among all CHDs (8). As a result, the quality of life of these patients is significantly impaired, especially in the physical well-being, with a strong correlation to their NYHA functional class (56).
For patients in the “grey zone” of PAH, i.e., pulmonary vascular resistance between 2.3 and 4.6 Wood Units (57), ASD closure remains under debate. Some patients have been successfully treated with sildenafil, bosentan or intravenous prostacyclin allowing for defect closure (58-60). However, randomized-controlled trials on vasodilator therapy before and after ASD closure in such physiological conditions are lacking. Therefore, CPET parameters (VO2max, VE/VCO2 slope) would probably be useful as primary or secondary outcomes in future clinical trials.
Atrial fibrillation (AF) is the most common arrhythmia associated with ASD. In a large cohort of 1,111 ASD patients diagnosed during childhood, Karunanithi et al. showed an significantly increased risk of AF; both with closure [adjusted hazard ratio (HR) 18.5; 95% CI: 7.8–44.1, P<0.0001] and without closure (HR 16.4; 95% CI: 6.8–39.8; P<0.0001), in comparison with controls. A comparison of surgical closure with transcatheter closure found no difference in terms of risk of AF (61). Classically, the AF needs to be reduced before CPET is performed. However, in case of chronic AF associated with ASD, CPET parameters are not specific, showing deterioration of peak VO2, oxygen pulse, VE/VCO2 slope, and heart rate response (62).
Abnormal heart rate response during exercise is uncommon in ASDs, however, chronotropic incompetence may occur after surgical repair and therefore be diagnosed by CPET (63). Moreover, ASDs may be associated to progressive atrioventricular block, related to NK2 homeobox 5 (NKX2.5) mutations (64).
Heart failure in ASDs may also be related to left ventricular (LV) dysfunction. Indeed, some studies have reported deterioration of systolic or diastolic LV function after ASD closure. An acute rise in the volume and filling pressure of both the left atrium and LV may cause left-sided heart failure, even without evident LV dysfunction prior to the intervention (65-67). This rare condition mostly concerns elderly patients and/or patients with a large shunt, for which impairment of LV contractility may occur until 6 months after closure (68). CPET results for LV dysfunction after ASD closure are not specific: decrease in peak VO2, oxygen pulse and anaerobic threshold, and increase in heart rate response and VE/VCO2 slope. Therefore, close long-term observation is required after ASD closure, especially in older patients with a large shunt. In a recent study, the absolute ASD shunt volume per minute remained unchanged under dobutamine stress test compared to values at rest, and the peak VO2 correlated to cardiac output but not to RV volume, suggesting abnormal LV compliance as a limiting factor for exercise capacity (69).
CPET in paediatric patients with ASDs
Exercise intolerance is uncommon in young children with an isolated ASD (28). However, pulmonary function is often impaired in this age group and improves after ASD closure (70). Although exercise capacity seems to insidiously decrease with age, serial CPET studies starting from paediatric age are clearly missing (24). In a small paediatric cohort (N=16), CPET parameters in children with ASD only slightly differ from those in normal children (71). Another small cohort (N=10) found no differences in terms of VO2max between children with ASD and controls (72). Similarly, in a study of 22 children with ASD surgical repair (N=22), Rosenthal et al. found that exercise performance was unaffected by age at repair (73).
Although peak VO2 correlates with the quality of life of children with CHDs (9), the follow-up of paediatric CHD patients with CPET is not yet recommended as it is in adults with CHDs (10). Nevertheless, a normal or sub-normal peak VO2 in such a simple CHD may participate in promoting self-confidence to the child, reassuring his or her family, and motivating them to engage the young patient in physical activity (74). Indeed, although physical activity and sports are in almost all cases authorized in children with ASDs (75), CHD children are often hovered over by their parents, stigmatized by their teachers, and eventually remain on the side-lines (74,76). Consequently, their quality of life is significantly reduced (9,77). Therefore, CPET follow-up in children with ASDs may detect early onset of muscular deconditioning, for which cardiac rehabilitation may be considered (75,78).
Conclusions
The CPET provides important information in assessment and follow-up of patients with ASDs, for both children and adults. In most cases, the exercise capacity is fairly normal and CPET contributes to promote sports participation. Furthermore, a regular CPET follow-up is necessary to evaluate the occurrence, severity and physiological mechanisms of comorbities, i.e., heart failure, pulmonary hypertension and arrhythmia. Finally, CPET follow-up in patients with ASDs may detect early onset of muscular deconditioning, for which cardiac rehabilitation may be considered.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wood PH. Appreciating the consequences of disease: the international classification of impairments, disabilities, and handicaps. WHO Chron 1980;34:376-80. [PubMed]
- Myers J, Arena R, Dewey F, et al. A cardiopulmonary exercise testing score for predicting outcomes in patients with heart failure. Am Heart J 2008;156:1177-83. [Crossref] [PubMed]
- Sue DY. Excess ventilation during exercise and prognosis in chronic heart failure. Am J Respir Crit Care Med 2011;183:1302-10. [Crossref] [PubMed]
- Guazzi M, Adams V, Conraads V, et al. EACPR/AHA scientific statement. clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012;126:2261-74. [Crossref] [PubMed]
- Diller GP, Dimopoulos K, Okonko D, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation 2005;112:828-35. [Crossref] [PubMed]
- Diller GP, Giardini A, Dimopoulos K, et al. Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J 2010;31:3073-83. [Crossref] [PubMed]
- Inuzuka R, Diller GP, Borgia F, et al. Comprehensive use of cardiopulmonary exercise testing identifies adults with congenital heart disease at increased mortality risk in the medium term. Circulation 2012;125:250-9. [Crossref] [PubMed]
- Kempny A, Dimopoulos K, Uebing A, et al. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life--single centre experience and review of published data. Eur Heart J 2012;33:1386-96. [Crossref] [PubMed]
- Amedro P, Picot MC, Moniotte S, et al. Correlation between cardio-pulmonary exercise test variables and health-related quality of life among children with congenital heart diseases. Int J Cardiol 2016;203:1052-60. [Crossref] [PubMed]
- Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57. [Crossref] [PubMed]
- van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241-7. [Crossref] [PubMed]
- Muta H, Akagi T, Egami K, et al. Incidence and clinical features of asymptomatic atrial septal defect in school children diagnosed by heart disease screening. Circ J 2003;67:112-5. [Crossref] [PubMed]
- Seldon WA, Rubinstein C, Fraser AA. The incidence of atrial septal defect in adults. Br Heart J 1962;24:557-60. [Crossref] [PubMed]
- Butera G, Biondi-Zoccai G, Sangiorgi G, et al. Percutaneous versus surgical closure of secundum atrial septal defects: a systematic review and meta-analysis of currently available clinical evidence. EuroIntervention 2011;7:377-85. [Crossref] [PubMed]
- Barron AJ, Wensel R, Francis DP, et al. The role for cardiopulmonary exercise testing in patients with atrial septal defects: a review. Int J Cardiol 2012;161:68-72. [Crossref] [PubMed]
- Durmała J, Rokicki W, Pilis W, et al. Physical fitness of children after cardiosurgical correction of an atrial septal defect type II. Przegl Lek 1998;55:378-81. [PubMed]
- Fritsch J, Winter UJ, Kaemmerer H, et al. Cardiopulmonary capacity of patients with congenital heart defects in childhood, adolescence and adulthood. Z Kardiol 1994;83:131-9. [PubMed]
- Forman DE, Myers J, Lavie CJ, et al. Cardiopulmonary exercise testing: relevant but underused. Postgrad Med 2010;122:68-86. [Crossref] [PubMed]
- Fredriksen PM, Veldtman G, Hechter S, et al. Aerobic capacity in adults with various congenital heart diseases. Am J Cardiol 2001;87:310-4. [Crossref] [PubMed]
- Suchoń E, Podolec P, Tomkiewicz-Pajak L, et al. Cardiopulmonary exercise capacity in adult patients with atrial septal defect. Przegl Lek 2002;59:747-51. [PubMed]
- Trojnarska O, Szyszka A, Gwizdala A, et al. Evaluation of exercise capacity with cardiopulmonary exercise testing and type B natriuretic peptide concentrations in adult patients with patent atrial septal defect. Cardiology 2006;106:154-60. [Crossref] [PubMed]
- Giardini A, Donti A, Formigari R, et al. Determinants of cardiopulmonary functional improvement after transcatheter atrial septal defect closure in asymptomatic adults. J Am Coll Cardiol 2004;43:1886-91. [Crossref] [PubMed]
- Van De Bruaene A, Buys R, Vanhees L, et al. Cardiopulmonary exercise testing and SF-36 in patients with atrial septal defect type secundum. J Cardiopulm Rehabil Prev 2011;31:308-15. [Crossref] [PubMed]
- Geva T, Martins JD, Wald RM. Atrial septal defects. Lancet 2014;383:1921-32. [Crossref] [PubMed]
- Brochu MC, Baril JF, Dore A, et al. Improvement in exercise capacity in asymptomatic and mildly symptomatic adults after atrial septal defect percutaneous closure. Circulation 2002;106:1821-6. [Crossref] [PubMed]
- Craig RJ, Selzer A. Natural history and prognosis of atrial septal defect. Circulation 1968;37:805-15. [Crossref] [PubMed]
- Suchon E, Tracz W, Podolec P, et al. Atrial septal defect in adults: echocardiography and cardiopulmonary exercise capacity associated with hemodynamics before and after surgical closure. Interact Cardiovasc Thorac Surg 2005;4:488-92. [Crossref] [PubMed]
- Rhodes J, Patel H, Hijazi ZM. Effect of transcatheter closure of atrial septal defect on the cardiopulmonary response to exercise. Am J Cardiol 2002;90:803-6. [Crossref] [PubMed]
- Nakanishi N, Yoshioka T, Fujii T, et al. Comparison of exercise capacity evaluated by cardiopulmonary exercise test and hemodynamic parameters in patients with atrial septal defect. Kokyu To Junkan 1992;40:789-95. [PubMed]
- Kobayashi Y, Nakanishi N, Kosakai Y. Pre- and postoperative exercise capacity associated with hemodynamics in adult patients with atrial septal defect: a retrospective study. Eur J Cardiothorac Surg 1997;11:1062-6. [Crossref] [PubMed]
- Helber U, Baumann R, Seboldt H, et al. Atrial septal defect in adults: cardiopulmonary exercise capacity before and 4 months and 10 years after defect closure. J Am Coll Cardiol 1997;29:1345-50. [Crossref] [PubMed]
- Van De Bruaene A, De Meester P, Buys R, et al. Right ventricular load and function during exercise in patients with open and closed atrial septal defect type secundum. Eur J Prev Cardiol 2013;20:597-604. [Crossref] [PubMed]
- Baydar O, Oktay V, Sinan UY, et al. Strain analysis during exercise in patients with asymptomatic atrial septal defect. Echocardiography 2014;31:1239-44. [Crossref] [PubMed]
- Poggio R, Arazi HC, Giorgi M, et al. Prediction of severe cardiovascular events by VE/VCO2 slope versus peak VO2 in systolic heart failure: a meta-analysis of the published literature. Am Heart J 2010;160:1004-14. [Crossref] [PubMed]
- Chaix MA, Marcotte F, Dore A, et al. Risks and benefits of exercise training in adults with congenital heart disease. Can J Cardiol 2016;32:459-66. [Crossref] [PubMed]
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in collaboration with the american society of echocardiography, heart rhythm society, international society for adult congenital heart disease, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol 2008;52:e143-263. [Crossref] [PubMed]
- Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 2013;128:873-934. [Crossref] [PubMed]
- Gierat-Haponiuk K, Haponiuk I, Szalewska D, et al. Effect of complex cardiac rehabilitation on physical activity and quality of life during long-term follow-up after surgical correction of congenital heart disease. Kardiol Pol 2015;73:267-73. [Crossref] [PubMed]
- Suchon E, Pieculewicz M, Tracz W, et al. Transcatheter closure as an alternative and equivalent method to the surgical treatment of atrial septal defect in adults: comparison of early and late results. Med Sci Monit 2009;15:CR612-7. [PubMed]
- Takaya Y, Taniguchi M, Akagi T, et al. Long-term effects of transcatheter closure of atrial septal defect on cardiac remodeling and exercise capacity in patients older than 40 years with a reduction in cardiopulmonary function. J Interv Cardiol 2013;26:195-9. [Crossref] [PubMed]
- Schoen SP, Kittner T, Bohl S, et al. Transcatheter closure of atrial septal defects improves right ventricular volume, mass, function, pulmonary pressure, and functional class: a magnetic resonance imaging study. Heart 2006;92:821-6. [Crossref] [PubMed]
- Giardini A, Donti A, Specchia S, et al. Long-term impact of transcatheter atrial septal defect closure in adults on cardiac function and exercise capacity. Int J Cardiol 2008;124:179-82. [Crossref] [PubMed]
- Komar M, Przewlocki T, Olszowska M, et al. The benefit of atrial septal defect closure in elderly patients. Clin Interv Aging 2014;9:1101-7. [Crossref] [PubMed]
- Jategaonkar S, Scholtz W, Schmidt H, et al. Cardiac remodeling and effects on exercise capacity after interventional closure of atrial septal defects in different adult age groups. Clin Res Cardiol 2010;99:183-91. [Crossref] [PubMed]
- Komar M, Przewlocki T, Olszowska M, et al. Is it worth closing the atrial septal defect in patients with insignificant shunt? Postepy Kardiol Interwencyjnej 2014;10:78-83. [Crossref] [PubMed]
- Lange SA, Braun MU, Schoen SP, et al. Latent pulmonary hypertension in atrial septal defect: Dynamic stress echocardiography reveals unapparent pulmonary hypertension and confirms rapid normalisation after ASD closure. Neth Heart J 2013;21:333-43. [Crossref] [PubMed]
- Kim JY, Yun BS, Lee S, et al. Changes in strain pattern and exercise capacity after transcatheter closure of atrial septal defects. Korean Circ J 2017;47:245-53. [Crossref] [PubMed]
- Jategaonkar S, Scholtz W, Schmidt H, et al. Percutaneous closure of atrial septal defects: echocardiographic and functional results in patients older than 60 years. Circ Cardiovasc Interv 2009;2:85-9. [Crossref] [PubMed]
- Heiberg J, Nyboe C, Hjortdal VE. Impaired ventilatory efficiency after closure of atrial or ventricular septal defect. Scand Cardiovasc J 2017;51:221-7. [Crossref] [PubMed]
- Menting ME, van den Bosch AE, McGhie JS, et al. Ventricular myocardial deformation in adults after early surgical repair of atrial septal defect. Eur Heart J Cardiovasc Imaging 2015;16:549-57. [Crossref] [PubMed]
- Schindler MB, Bohn DJ, Bryan AC, et al. Increased respiratory system resistance and bronchial smooth muscle hypertrophy in children with acute postoperative pulmonary hypertension. Am J Respir Crit Care Med 1995;152:1347-52. [Crossref] [PubMed]
- Yau KI, Fang LJ, Wu MH. Lung mechanics in infants with left-to-right shunt congenital heart disease. Pediatr Pulmonol 1996;21:42-7. [Crossref] [PubMed]
- Rabinovitch M, Keane JF, Norwood WI, et al. Vascular structure in lung tissue obtained at biopsy correlated with pulmonary hemodynamic findings after repair of congenital heart defects. Circulation 1984;69:655-67. [Crossref] [PubMed]
- Santos M, Systrom D, Epstein SE, et al. Impaired exercise capacity following atrial septal defect closure: an invasive study of the right heart and pulmonary circulation. Pulm Circ 2014;4:630-7. [Crossref] [PubMed]
- De Meester P, Van De Bruaene A, Herijgers P, et al. Increased pulmonary artery pressures during exercise are related to persistent tricuspid regurgitation after atrial septal defect closure. Acta Cardiol 2013;68:365-72. [Crossref] [PubMed]
- Amedro P, Basquin A, Gressin V, et al. Health-related quality of life of patients with pulmonary arterial hypertension associated with CHD: the multicentre cross-sectional ACHILLE study. Cardiol Young 2016;26:1250-9. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): endorsed by: association for European paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]
- Kim YH, Yu JJ, Yun TJ, et al. Repair of atrial septal defect with Eisenmenger syndrome after long-term sildenafil therapy. Ann Thorac Surg 2010;89:1629-30. [Crossref] [PubMed]
- Schwerzmann M, Zafar M, McLaughlin PR, et al. Atrial septal defect closure in a patient with "irreversible" pulmonary hypertensive arteriopathy. Int J Cardiol 2006;110:104-7. [Crossref] [PubMed]
- Frost AE, Quinones MA, Zoghbi WA, et al. Reversal of pulmonary hypertension and subsequent repair of atrial septal defect after treatment with continuous intravenous epoprostenol. J Heart Lung Transplant 2005;24:501-3. [Crossref] [PubMed]
- Karunanithi Z, Nyboe C, Hjortdal VE. Long-term risk of atrial fibrillation and stroke in patients with atrial septal defect diagnosed in childhood. Am J Cardiol 2017;119:461-5. [Crossref] [PubMed]
- Zakeri R, Borlaug BA, McNulty SE, et al. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail 2014;7:123-30. [Crossref] [PubMed]
- Heiberg J, Nyboe C, Hjortdal VE. Permanent chronotropic impairment after closure of atrial or ventricular septal defect. Scand Cardiovasc J 2017;51:271-6. [Crossref] [PubMed]
- Xu YJ, Qiu XB, Yuan F, et al. Prevalence and spectrum of NKX2.5 mutations in patients with congenital atrial septal defect and atrioventricular block. Mol Med Rep 2017;15:2247-54. [Crossref] [PubMed]
- Ewert P, Berger F, Nagdyman N, et al. Masked left ventricular restriction in elderly patients with atrial septal defects: a contraindication for closure? Catheter Cardiovasc Interv 2001;52:177-80. [Crossref] [PubMed]
- Schubert S, Peters B, Abdul-Khaliq H, et al. Left ventricular conditioning in the elderly patient to prevent congestive heart failure after transcatheter closure of atrial septal defect. Catheter Cardiovasc Interv 2005;64:333-7. [Crossref] [PubMed]
- Masutani S, Taketazu M, Mihara C, et al. Usefulness of early diastolic mitral annular velocity to predict plasma levels of brain natriuretic peptide and transient heart failure development after device closure of atrial septal defect. Am J Cardiol 2009;104:1732-6. [Crossref] [PubMed]
- Yoo BW, Kim JO, Eun LY, et al. Time course of the changes in right and left ventricle function and associated factors after transcatheter closure of atrial septal defects. Congenit Heart Dis 2018;13:131-9. [PubMed]
- Stephensen SS, Steding-Ehrenborg K, Thilen U, et al. Changes in blood volume shunting in patients with atrial septal defects: assessment of heart function with cardiovascular magnetic resonance during dobutamine stress. Eur Heart J Cardiovasc Imaging 2017;18:1145-52. [Crossref] [PubMed]
- Lee YS, Jeng MJ, Tsao PC, et al. Pulmonary function changes in children after transcatheter closure of atrial septal defect. Pediatr Pulmonol 2009;44:1025-32. [Crossref] [PubMed]
- Pfammatter JP, Zanolari M, Schibler A. Cardiopulmonary exercise parameters in children with atrial septal defect and increased pulmonary blood flow: short-term effects of defect closure. Acta Paediatr 2002;91:65-70. [Crossref] [PubMed]
- Perrault H, Drblik SP, Montigny M, et al. Comparison of cardiovascular adjustments to exercise in adolescents 8 to 15 years of age after correction of tetralogy of fallot, ventricular septal defect or atrial septal defect. Am J Cardiol 1989;64:213-7. [Crossref] [PubMed]
- Rosenthal M, Redington A, Bush A. Cardiopulmonary physiology after surgical closure of asymptomatic secundum atrial septal defects in childhood. Exercise performance is unaffected by age at repair. Eur Heart J 1997;18:1816-22. [Crossref] [PubMed]
- O'Byrne ML, Mercer-Rosa L, Ingall E, et al. Habitual exercise correlates with exercise performance in patients with conotruncal abnormalities. Pediatr Cardiol 2013;34:853-60. [Crossref] [PubMed]
- Takken T, Giardini A, Reybrouck T, et al. Recommendations for physical activity, recreation sport, and exercise training in paediatric patients with congenital heart disease: a report from the Exercise, Basic & translational research section of the European association of cardiovascular prevention and rehabilitation, the European congenital heart and lung exercise group, and the association for European paediatric cardiology. Eur J Prev Cardiol 2012;19:1034-65. [Crossref] [PubMed]
- Moola F, Fusco C, Kirsh JA. The perceptions of caregivers toward physical activity and health in youth with congenital heart disease. Qual Health Res 2011;21:278-91. [Crossref] [PubMed]
- Amedro P, Dorka R, Moniotte S, et al. Quality of life of children with congenital heart diseases: a multicenter controlled cross-sectional study. Pediatr Cardiol 2015;36:1588-601. [Crossref] [PubMed]
- Gomes-Neto M, Saquetto MB. Impact of exercise training in aerobic capacity and pulmonary function in children and adolescents after congenital heart disease surgery: a systematic review with meta-analysis. Pediatr Cardiol 2016;37:217-24. [Crossref] [PubMed]


