Pulmonary embolism concurrent with lung cancer and central emboli predict mortality in patients with lung cancer and pulmonary embolism
Introduction
Cancer is one of the important risk factors for venous thromboembolism (VTE). VTE occurs two to four times more frequently in patients with cancer than in those without cancer (1,2). In a study using a Medicare database, malignancies with the highest risk of VTE when adjusted for prevalence included ovarian cancer, brain tumors, and pancreatic cancer (3), whereas in a Dutch population-based study, hematologic malignancies were associated with the highest risk of VTE, followed by lung and gastrointestinal cancers (4). Lung cancer is the most commonly identified malignancy in patients with VTE (5), with an incidence of 3–13.9% in patients with VTE and that of up to 3.8% in patients with pulmonary embolism (PE) (6-9).
The mechanisms of VTE in cancer patients, although not completely established, appear to be multifactorial (6,7). The occurrence of VTE in lung cancer has a prognostic implication, in that the survival of lung cancer patients with PE was shorter than that of those without PE (10). However, most deaths in patients with lung cancer and PE or VTE have been attributed to lung cancer rather than thromboembolism (11). These findings suggest that VTE may be a marker of an advanced stage cancer or of a more biologically aggressive tumor (12). A previous study including small numbers of patients demonstrated that patients with lung cancer with concurrently diagnosed PE had poorer survival than those without PE (10). In another study, no difference in survival was found between patients with PE diagnosed at less than 3 months after the diagnosis of lung cancer and those with PE diagnosed at 3 months or more following lung cancer diagnosis (13). Therefore, it is unclear whether the timing of the presentation of PE in patients with lung cancer may influence patient prognosis. Several factors that may determine short-term prognosis in patients with PE, including pulmonary embolism severity index (PESI) score, cardiac biomarkers, right ventricular (RV) dilation, and central emboli, have been proposed (14). However, information regarding these PE-related prognostic factors in patients with lung cancer and PE is lacking. The aim of the present study was to examine the clinical features of patients with lung cancer patients and PE, to compare clinical characteristics of PE found concurrently at lung cancer diagnosis and PE developed sequentially after the diagnosis of lung cancer, and to investigate the PE-related predictors of mortality in patients with lung cancer patients and PE.
Methods
Study design
The present study was retrospectively conducted at two university-affiliated hospitals [Kyungpook National University Hospital (KNUH) and Kyungpook National University Medical Center (KNUMC)] in Daegu, Korea. Patients with active lung cancer with PE, which had been diagnosed by computed tomography (CT), were enrolled. Active lung cancer was defined based on fulfillment of one of the following conditions: (I) detection of lung cancer within 6 months after PE diagnosis; (II) current therapy of supportive care or active anticancer treatment for lung cancer; (III) within 6 months after the end of anticancer therapy for lung cancer. The patients with lung cancer and PE were categorized into a group of patients with PE diagnosed concomitantly with lung cancer (concurrent group) and a group with PE detected after the diagnosis of lung cancer (sequential group). To identify patients with lung cancer patients and PE, we reviewed the database of patients with PE (14), searched electronic medical records under the diagnosis codes, and performed a search of CT readings of the two hospitals with the search terms of “lung cancer” and “pulmonary embolism” or “pulmonary thromboembolism” from January 2005 through December 2014 at KNUH and from January 2011 through December 2014 at KNUMC. Exclusion criteria were the following: (I) in situ pulmonary artery thrombosis (15); (II) pulmonary artery obstruction by tumor mass (tumor emboli); (III) lack of medical records; (IV) lack of available CT with interpretable quality images. This study was approved by the Institutional Review Board of each hospital (KNUH IRB 2016-09-020 and KNUMC 2016-10-005), which waived the requirement for written informed consent because of the retrospective nature of the study.
Data collection
Demographic data, symptoms, comorbid conditions, and risk factors for VTE were reviewed. PE was determined as unprovoked when no reversible provoking risk factors, such as surgery, trauma, pregnancy and puerperium within 3 months of the event, or immobilization (bed rest within the previous month for most of the day for ≥3 consecutive days) existed (16). The PESI score was retrospectively calculated (17). PE-related outcomes, including a PE-related in-hospital mortality, adverse outcomes, and VTE recurrence were assessed. An adverse outcome comprised PE-related in-hospital death and serious clinical conditions due to PE, including requirement for inotropic support, impending respiratory failure or mechanical ventilation, cardiopulmonary resuscitation, and secondary thrombolysis (18). A PE-related in-hospital death was defined if an in-hospital death was directly caused by PE, or if it could not be attributed to other causes and PE could not be excluded (18). Blood laboratory data, including serum N-terminal-pro-B-type natriuretic peptide (NT-proBNP) and plasma troponin I, were also assessed.
Lung cancer-related data, including time between the diagnosis of lung cancer and PE detection, histologic type, initial stage, stage at the diagnosis of PE, chemotherapy, and survival data, were reviewed. The stage of lung cancer was determined as stage I, II, III, or IV according to the seventh edition of the TNM classification developed by the International Association for the Study of Lung Cancer in 2009 (19), and pathologic stage was adopted in patients who underwent surgical resection.
Radiological evaluation
The diagnosis of PE was made on CT images as a sharply delineated pulmonary arterial filling defect in at least two consecutive sections, either located centrally within the vessel or with acute angles at its interface with the vessel wall (20). The largest pulmonary arteries in which pulmonary emboli were located were determined, and central PE comprised the right or left pulmonary artery or a more proximal location. The diameters of the RV and left ventricular (LV) were measured at their widest points between the inner surface of the free wall and the surface of the interventricular septum (21), and the RV/LV diameter ratios were calculated. An RV/LV diameter ratio of ≥1 was designated as RV dilation (18). Pulmonary infarction was defined as a peripheral consolidation in the region of pulmonary emboli, based on the modified criteria of a previous study (22).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows version 22.0 (IBM Corp., Armonk, NY, USA). P values <0.05 were considered statistically significant. Data were expressed as means ± standard deviations (SDs) or medians [interquartile ranges (IQRs)] for non-normally distributed continuous variables and as numbers and percentages for categorical variables. Continuous variables were compared using Student’s t-test or the Mann-Whitney U test if non-normally distributed, whereas categorical variables were compared using the Chi-squared test or Fisher’s exact test. Survival was analyzed using the Kaplan-Meier method. Independent prognostic factors for survival were determined using a Cox proportional hazards model.
Results
Clinical characteristics with regard to PE
Overall, 5,005 patients with lung cancer were identified, and 267 patients with lung cancer and PE (5.3%) were included in the present study. Baseline characteristics of the patients are presented in Table 1. Patients’ median age at the diagnosis of PE was 69 years (IQR, 63–84 years), and there was a higher proportion with men (65.2%). The most common risk factor for VTE, other than lung cancer, was chemotherapy [177 patients (66.3%)], followed by immobilization [14 patients (5.2%)]. Unsuspected PE was found in more than half (53.6%) of the patients. Most patients [220 (82.4%)] underwent anticoagulation therapy, and the reasons for lack of anticoagulation therapy were as follows: pulmonary emboli overlooked by attending physicians, n=19 (40.4%); contraindications to anticoagulation, n=3 (6.4%); refusal to receive anticoagulation, n=3 (6.4%); and unknown causes, n=22 (46.8%). PE-related in-hospital deaths, adverse outcomes, and recurrent VTE occurred in 2 (0.7%), 8 (3.0%), and 12 patients (4.5%), respectively. On CT scan, 48 patients (18.0%) had central PE, and RV dilation was noted in 55 patients (20.6%). Pulmonary infarction was observed in 18 patients (6.7%), and pleural effusion in 39 (14.6%).
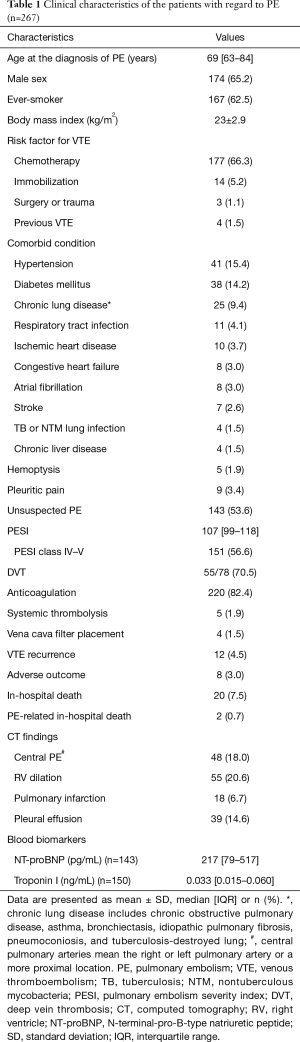
Full table
Clinical characteristics with regard to lung cancer
Data concerning lung cancer are detailed in Table 2. The median time between the diagnosis of lung cancer and the detection of PE was 135 days (range, 69–320 days). PE developed before or at the time of diagnosis of lung cancer in 27 patients (concurrent group, 10.1%), and in the remaining patients, PE occurred after the diagnosis of lung cancer (sequential group). The most common histologic type of lung cancer was adenocarcinoma [140 (52.4%)], followed by squamous cell carcinoma [53 (19.9%)] and small cell carcinoma [50 (18.7%)]. When lung cancer was diagnosed, most patients (85.0%) had stage IV [160 (59.9%)] or III [67 (25.1%)] cancer. The median survival after the diagnosis of lung cancer was 448 days (range, 255–774 days) and the median survival after the diagnosis of PE was 207 days (range, 88–485 days). Of the 129 patients whose causes of death were identified, progression of lung cancer [121 (93.8%)] was the most common.
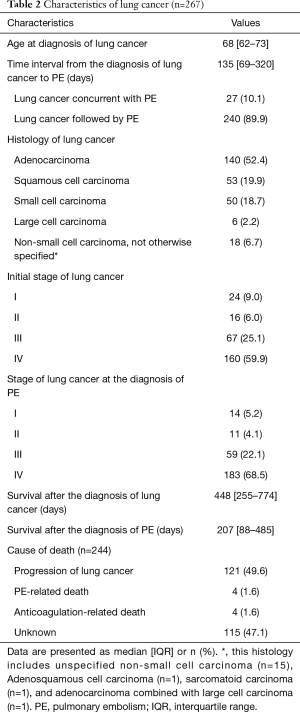
Full table
Comparisons between the concurrent and sequential groups
As noted above, all patients were categorized into either the concurrent or sequential group, and clinical parameters were compared between the two groups (Table 3). In the concurrent group, risk factors for VTE including immobilization [4 (14.8%) vs. 10 (4.2%), P=0.042], surgery/trauma [2 (7.4%) vs. 1 (0.4%), P=0.028], and previous VTE [2 (7.4%) vs. 2 (0.8%), P=0.052] were more common than in the sequential group, whereas chemotherapy was administered in 177 (73.8%) of the patients in the sequential group. Of the 50 patients with small cell carcinoma, PE developed sequentially after the diagnosis of lung cancer in 49 patients, of whom 45 underwent chemotherapy, with the other one having a predisposing condition of immobilization. RV dilation was significantly more common in the concurrent group [14 (51.9%) vs. 41 (17.1%), P<0.001] than in the sequential group, whereas the frequency of central PE was not significantly different between the two groups (Table 4). Systemic thrombolysis [2 (7.4%) vs. 3 (1.3%), P=0.081] and vena cava filter placement [3 (11.1%) vs. 1 (0.4%), P=0.003] were also more commonly performed in the concurrent group than in the sequential group. The median survival after the diagnosis of lung cancer was significantly shorter in the concurrent group than in the sequential group [214 (IQR, 19–409) vs. 498 (IQR, 430–566) days, P<0.001].
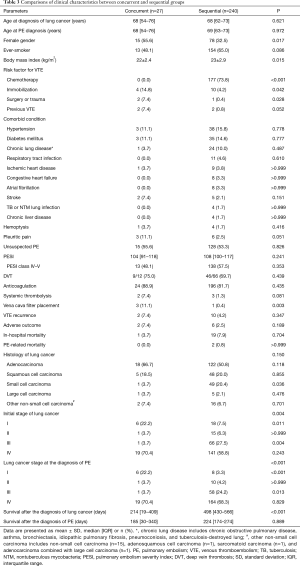
Full table

Full table
Survival analysis according to PE-related factors
Concurrence of lung cancer and PE (concurrent group vs. sequential group), presence vs. absence of anticoagulation, unsuspected vs. suspected PE, high PESI score (class IV–V vs. class I–III), central PE, and RV dilation were chosen as candidate PE-related prognostic factors that could affect the survival of patients with lung cancer (Figure 1). When adjusted for age, gender, smoking status (ever-smoker vs. never-smoker), stage (I–IV), and histologic type (small cell lung cancer vs. non-small cell lung cancer), concurrence of lung cancer and PE [hazard ratio (HR) =2.64, 95% confidence interval (CI): 1.57–4.43, P<0.001] and central PE (HR =1.46, 95% CI: 1.02–2.10, P=0.04) independently influenced the survival of patients with lung cancer and PE.
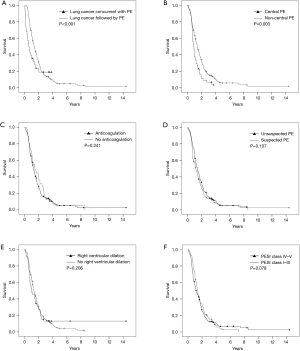
Discussion
The present study demonstrated that in patients with lung cancer and PE, the most common predisposing factors for PE after lung cancer alone was chemotherapy. The median time from the diagnosis of lung cancer to PE was 4.5 months, and approximately 10% of patients developed PE concurrently with lung cancer. The concurrent group was characterized by more common association with risk factors for VTE (immobilization, surgery or trauma and previous VTE), stage I lung cancer, and RV dilation than the sequential group. Nearly all patients with small cell lung cancer developed PE sequentially to lung cancer rather than concurrently. Of the PE-related parameters, concurrent diagnosis of PE in lung cancer and central PE were independent predictors of death in patients with lung cancer and PE, suggesting the timing of PE presentation and the size of emboli were important factors determining prognosis in these patients.
The most important clinical factors determining the rates of VTE in patients with lung cancer are stage and tumor histology (6). As expected, in the present study, most patients had advanced stage lung cancer. A hypercoagulable state in cancer has been commonly attributed to the production of mucin and overexpression of tissue factor (23). Mucin-producing adenocarcinomas of the lung are associated with increased risk of VTE (12). This finding can be explained by the concept that mucin may cause procoagulant secretion (24) and activate platelets and induce formation of microthrombi in the microvasculature (25). A previous study demonstrated that patients with adenocarcinoma of the lung had a higher risk of VTE than patients with squamous cell carcinoma, after adjusting for therapy and distant metastasis (25). Likewise, in the present study, the most common histology was adenocarcinoma, followed by squamous cell carcinoma and small cell carcinoma. Interestingly, in small cell lung cancer, almost all emboli, except for one, developed sequentially after the diagnosis of lung cancer, suggesting that PE is more likely to be provoked by chemotherapy rather than lung cancer itself. A recent study showed that cisplatin-based chemotherapy might be a strong predictor for the risk of thromboembolic events in small cell lung cancer (26). The most common risk factor for PE in patients with lung cancer was chemotherapy, in accordance with previous reports that chemotherapy increases the risk of VTE in patients with cancer (1-6), with a three-fold increased risk in lung cancer (25). The mechanism underlying the manner in which chemotherapy contributes to VTE risk has not been established. However, release of procoagulants and cytokines from cancer cells, direct endothelial damage, and down regulation of endogenous anticoagulants are likely to be involved in the pathogenesis of VTE in patients with cancer (27).
Patients with lung cancer and concurrent PE constituted approximately 10% of all patients with lung cancer and PE in the present study. The concurrent group had higher percentages of risk factors for VTE not directly related to lung cancer (immobilization, surgery or trauma, previous VTE). Thus, the possibility that provoking risk factors for VTE might contribute to the development of PE cannot be excluded. As reported in previous studies (6,11), most patients with PE had advanced stage lung cancer in the present study. However, early stage (stage I) lung cancer was significantly more common and stage III was significantly less common in the concurrent group. Furthermore, although anti-cancer therapy such as chemotherapy is an important risk factor for VTE in patients with lung cancer, it did not influence the occurrence of PE in the concurrent group. These findings suggest that prothrombotic activity of tumor cells plays a more important role in the occurrence of PE in the concurrent group. Compared with the sequential group, systemic thrombolysis and vena cava filter placement were more commonly used in the concurrent group: all patients had stage IV lung cancer. In addition, the concurrent group exhibited higher rates of RV dilation. These results imply that the concurrent group was associated with a more severe PE. In contrast, the frequency of central PE in the concurrent group did not differ from that in the sequential group. This can be partly explained by the finding that multiple lobar artery involvement by PE was significantly more common in the concurrent group than in the sequential group [10/12 (83.3%) vs. 17/79 (21.5%), P<0.001].
Concurrence of lung cancer with PE and central PE were significant prognostic factors for patients with lung cancer and PE. In the present study, in comparison with PE sequential to lung cancer, lung cancer concurrent with PE demonstrated reduced survival. Cancer patients who develop VTE have a higher mortality compared to those without VTE and with the same stage (23), suggesting that VTE is a marker for a more biologically aggressive tumor (12). From this point of view, we speculate that lung cancer concurrent with PE had more biologically aggressive tumor behavior than lung cancer with sequential PE (12). Although both RV dilation and central emboli were significant markers for a short-term prognosis in PE (14), central PE, not RV dilation, was an independent predictor of mortality in patients with lung cancer and PE. This finding can be explained by the notion that the size of emboli or the clot burden is more likely to contribute to the prognosis in these patients, rather than RV dysfunction. A previous study showed that anticoagulation therapy for unsuspected PE was associated with increased overall survival in patients with lung cancer (13). In contrast, whether patients with lung cancer and PE underwent anticoagulation or not did not affect the survival of these patients in the present study. This discrepancy can be partially explained by the finding that patients who did not receive anticoagulation had significantly higher proportion of peripheral PE (segmental or subsegmental) than patients who underwent anticoagulation [35/47 (74.5%) vs. 92/220 (41.8%), P<0.001].
Several limitations of the present study should be mentioned. First, selection bias was unavoidable due to the retrospective nature of the study. Although we searched the database of patients with PE, electronic medical records under the diagnosis codes, and CT readings, the possibility that some patients were omitted from selection could not be excluded. Second, missing laboratory data, such as NT-proBNP levels, could have influenced our results. Third, the fact that PE-specific therapy, such as anticoagulation, determined on clinician judgment may have affected the clinical outcome. Lastly, the causes of deaths were not identified in approximately half of the patients.
Conclusions
Lung cancer with concurrent PE, found in approximately 10% of all patients with lung cancer and PE, was associated with more severe PE and infrequent small cell carcinoma. The timing of PE presentation (concurrence of lung cancer and PE) and the size of emboli (central PE) were independent predictors of death in patients with lung cancer patients and PE.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of each hospital (KNUH IRB 2016-09-020 and KNUMC 2016-10-005), which waived the requirement for written informed consent because of the retrospective nature of the study.
References
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000;160:809-15. [Crossref] [PubMed]
- Rosendaal FR. Risk factors for venous thrombosis: prevalence, risk, and interaction. Semin Hematol 1997;34:171-87. [PubMed]
- Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine 1999;78:285-91. [Crossref] [PubMed]
- Blom JW, Doggen CJ, Osanto S, et al. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005;293:715-22. [Crossref] [PubMed]
- Sørensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846-50. [Crossref] [PubMed]
- Vitale C, D'Amato M, Calabro P, et al. Venous thromboembolism and lung cancer: a review. Multidiscip Respir Med 2015;10:28. [Crossref] [PubMed]
- Tesselaar ME, Osanto S. Risk of venous thromboembolism in lung cancer. Curr Opin Pulm Med 2007;13:362-67. [Crossref] [PubMed]
- Tagalakis V, Levi D, Agulnik JS, et al. High risk of deep vein thrombosis in patients with non-small cell lung cancer: a cohort study of 493 patients. J Thorac Oncol 2007;2:729-34. [Crossref] [PubMed]
- Connolly GC, Dalal M, Lin J, et al. Incidence and predictors of venous thromboembolism among ambulatory patients with lung cancer. Lung Cancer 2012;78:253-58. [Crossref] [PubMed]
- Chuang YM, Yu CJ. Clinical characteristics and outcomes of lung cancer with pulmonary embolism. Oncology 2009;77:100-6. [Crossref] [PubMed]
- Lee JW, Cha SI, Jung CY, et al. Clinical course of pulmonary embolism in lung cancer patients. Respiration 2009;78:42-8. [Crossref] [PubMed]
- Chew HK, Davies AM, Wun T, et al. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost 2008;6:601-8. [Crossref] [PubMed]
- Sun JM, Kim TS, Lee J, et al. Unsuspected pulmonary emboli in lung cancer patients: the impact on survival and the significance of anticoagulation therapy. Lung Cancer 2010;69:330-6. [Crossref] [PubMed]
- Choi KJ, Cha SI, Shin KM, et al. Central emboli rather than saddle emboli predict adverse outcomes in patients with acute pulmonary embolism. Thromb Res 2014;134:991-6. [Crossref] [PubMed]
- Cha SI, Choi KJ, Shin KM, et al. Clinical characteristics of in-situ pulmonary artery thrombosis in Korea. Blood Coagul Fibrinolysis 2015;26:903-7. [Crossref] [PubMed]
- Choi KJ, Cha SI, Shin KM, et al. Prevalence and predictors of pulmonary embolism in Korean patients with exacerbation of chronic obstructive pulmonary disease. Respiration 2013;85:203-9. [Crossref] [PubMed]
- Aujesky D, Roy PM, Le Manach CP, et al. Validation of a model to predict adverse outcomes in patients with pulmonary embolism. Eur Heart J 2006;27:476-81. [Crossref] [PubMed]
- Choi KJ, Cha SI, Shin KM, et al. Prognostic implications of computed tomographic right ventricular dilation in patients with acute pulmonary embolism. Thromb Res 2014;133:182-6. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Gladish GW, Choe DH, Marom EM, et al. Incidental pulmonary emboli in oncology patients: prevalence, CT evaluation, and natural history. Radiology 2006;240:246-55. [Crossref] [PubMed]
- Araoz PA, Gotway MB, Harrington JR, et al. Pulmonary embolism: prognostic CT findings. Radiology 2007;242:889-97. [Crossref] [PubMed]
- Cha SI, Shin KM, Lee J, et al. Clinical relevance of pulmonary infarction in patients with pulmonary embolism. Thromb Res 2012;130:e1-5. [Crossref] [PubMed]
- Corrales-Rodriguez L, Blais N. Lung cancer associated venous thromboembolic disease: a comprehensive review. Lung Cancer 2012;75:1-8. [Crossref] [PubMed]
- Loreto MF, De Martinis M, Corsi MP, et al. Coagulation and cancer: implications for diagnosis and management. Pathol Oncol Res 2000;6:301-12. [Crossref] [PubMed]
- Blom JW, Osanto S, Rosendaal FR. The risk of a venous thrombotic event in lung cancer patients: higher risk for adenocarcinoma than squamous cell carcinoma. J Thromb Haemost 2004;2:1760-5. [Crossref] [PubMed]
- Lee YG, Lee E, Kim I, et al. Cisplatin-based chemotherapy is a strong risk factor for thromboembolic events in small-cell lung cancer. Cancer Res Treat 2015;47:670-5. [Crossref] [PubMed]
- Donati MB, Falanga A. Pathogenetic mechanisms of thrombosis in malignancy. Acta Haematol 2001;106:18-24. [Crossref] [PubMed]


