Chinese multi-institutional registry (CMIR) for resected non-small cell lung cancer: survival analysis of 5,853 cases
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide (1). Surgical resection remains the standard of care and provides the only curable option for most patients with non-metastatic non-small cell lung cancer (NSCLC) (2). Study of the clinical data is essential in addressing the increasing clinical concerns in an evidence-based way and in further improving the clinical practice. However, no nationwide collaboration or database for NSCLC in China had been established until 2008. Therefore, previous publications from China on NSCLC surgery were predominantly small sample single-center or regional experience, which might introduce significant biases (3). In order to investigate insightfully the current situation of surgical treatment especially minimally invasive surgery for NSCLC in China during the first decade of this century as well as to make the full use of the abundant patient source, our center initiated and have completed a collaboration of establishing a multi-institutional registry for resected NSCLC. Herein, we sought to provide an overview of this database (Chinese Multi-institutional Registry, CMIR) and to raise a project for further collaboration.
Data collection and maintenance
A multi-institutional registry for 5,853 patients between January 2001 and December 2008 in seven institutions from the People’s Republic of China was established. The inclusion criteria were patients diagnosed with stage I to III NSCLC who underwent radical resection with systematic lymph node dissection or sampling. Patients who underwent resection for metastatic disease were excluded. Ethical approval was obtained from participating institutions through their respective institutional review boards or the chairperson of the ethics committee, who waived the need for patient consent for the study, as individual patients were not identified.
A blinded standardized data form was created to retrieve all relevant information on clinical data (age, sex, date of surgery, smoking history and forced expiratory volume in one second); pathologic data [histological type, pathological tumor (T), node (N) and metastasis (M) status]; treatment-related data (type of resection-lobectomy, sleeve resection versus pneumonectomy, VATS versus open procedure) and perioperative outcomes. Standardized clinical data for consecutive patients treated in each of the seven institutions were entered into an independent central database at the Baird Institute for Applied Heart and Lung Surgical Research in Sydney, Australia. Follow-up data for all patients was obtained from their most recent medical review, which consisted of clinical examination and assessment of chest X-rays or computed tomography scans. Pathologic staging was characterized according to the seventh edition of the AJCC TNM Classification. An independent biostatistician team was authorized to manage and analyze the collected data. In this report, the survival curves were generated by Kaplan-Meier algorithm (4) and the overall survival rates were estimated through Life Table method (5). All calculations were conducted in SPSS 13.0 for Windows (SPSS, Chicago, IL, USA).
Overview of the database
Data were collected from thoracic surgery departments of the following seven medical centers in China (illustrated in Figure 1): the First Affiliated Hospital of Guangzhou Medical University (Guangzhou), Shanghai Pulmonary Hospital of Tongji University (Shanghai), Shanghai Zhongshan Hospital of Fudan University (Shanghai), West China Hospital of Sichuan University (Chengdu), China and Japan Friendship Hospital (Beijing), Shenzhen People’s Hospital (Shenzhen), Cancer Center of Sun Yat-Sen University (Guangzhou).
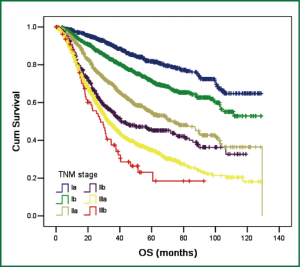
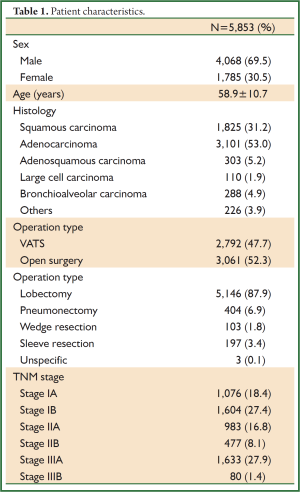
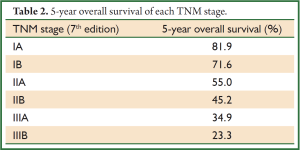
A total of 5,853 cases from 2001 to 2008 were reviewed and entered the database. The average age of patients at diagnosis was 58.9±10.7 years. Males (4,068/5,853, 59.5%) and adenmocarcinoma (3,101/5,853, 53.0%) represented the majority of all cases. Nearly half of patients (2,792/5,853, 47.7%) in this database underwent VATS. Lobectomy (5,146/5,853, 87.9%) was still the mainstream operation types in practice. Table 1 summarized some patient characteristics. By using Kaplan-Meier and Life Table methods, we measured the overall survival of these patients. As survival curves showed, patients in different TNM stage had distinct overall survival (P<0.001, Figure 2). The 5-year overall survival rates were listed in Table 2 and the survival rates were concordantly lower as the stage increased.
Full table
Full table
Discussion
To our knowledge, this is the first and the largest multi-institutional data collaboration for resected NSCLC in China. Participated institutions are all medical centers leading the field of thoracic surgery in developed Chinese cities including Beijing, Shanghai, Guangzhou, Shenzhen and Chengdu, representing the most advanced technical standards and medical care conditions in China (6). Patients from any of the above centers received standard treatments and were well documented in a consecutive way.
Despite some missing values associated with the retrospective nature of collection, optimal data quality was obtained. Both patient features and outcomes of survival analyses were concordant with the currently local conditions. Notably, this database contained patients from the period of 2000s in which VATS became increasingly accepted and popular in developed areas of China since it was first introduced into China by our center at 1994 (7). Based on CMIR, we have published a series of literatures since 2009 (8-10) and more upcoming reports are being processed. In addition, the database is being well maintained by the coordinators and being updated at regular intervals.
Being aware that large sample size is essential in publishing high impact studies, we do feel the need to expand and share this database with our colleagues. Based on what have been established, we are working on setting up a project for extended collaboration.
The principle rule is that those who contribute a certain amount of qualified data to CMIR could earn the right for secondary use of the whole data set to verify a hypothesis or validate a result. Any data donator may initiate a study plan by submitting a proposal to the database management committee for scientific evaluation. With the approval from the committee, the statistician team will analyze the data and provide a report of relevant results to the initiators. The manuscript will be finished and be submitted to the journal by the initiators after being reviewed by the committee. Certainly, authorship of the manuscript is determined by the initiators with all data contributors being acknowledged.
In addition to the retrospective collection, prospective records are being built in our center recent years. Thus, both retrospectively and prospectively collected data are welcomed, being entered into different data sets respectively. Through these efforts, we aim at establishing extensive nationwide data collaboration and expect more publications regarding NSCLC on high impact international journals from mainland China.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in OncologyTM. Non-Small Cell Lung Cancer (2013. V2).
- Berk RA. An Introduction to Sample Selection Bias in Sociological Data. American Sociological Review 1983;48:386-98.
- Kleinbaum DG. eds. Kaplan-Meier survival curves and the log-rank test in survival analysis: A self learning text (ed 2). New York, NY: Springer Verlag, 1997:45-76.
- Cutler SJ, Ederer F. Maximum utilization of the life table method in analyzing survival. J Chronic Dis 1958;8:699-712. [PubMed]
- Hospital management institute, Fudan University. Best hospitals and the best specialist reputation rankings of China 2012. Available online: http://www.fudanmed.com/institute/
- Li G. Minimally invasive thoracic surgery: news from the 3rd Asian-Pacific VATS Performance & the 6th China Lung Cancer MITS Forum. J Thorac Dis 2012;4:681-7.
- Cao C, Zhu ZH, Yan TD, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: a propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg 2013;44:849-54. [PubMed]
- Xiong X, Shao W, Yin W, et al. Video-assisted thoracoscopic surgery for stage I non-small cell lung cancer: long-term survival and prognostic factors. Tumour Biol 2013. [Epub ahead of print]. [PubMed]
- He J, Shao W, Cao C, et al. Long-term outcome and cost-effectiveness of complete versus assisted video-assisted thoracic surgery for non-small cell lung cancer. J Surg Oncol 2011;104:162-8. [PubMed]


