Esophageal cancer in elderly patients: a population-based study
Introduction
Esophageal cancer (EC) is one of the most common cancers worldwide, with approximately 455,800 new EC cases and 400,200 deaths occurring in 2012 (1). In the United States, it was estimated that 16,980 people were diagnosed with EC and that 15,590 deaths occurred in 2015 (2). Although there have been many recent advances in diagnosis and therapy, the 5-year survival rate for EC ranges from 15% to 25% (3).
The impact of EC on elderly patients is increasing because of the aging population. Several studies (4,5) and a meta-analysis (6) demonstrated that, compared to younger patients, patients 70 years of age and older undergoing surgical resection for EC had lower survival rates. However, no significant differences were observed in survival between elderly and younger patients after esophageal resection in some studies (7-11), suggesting that advanced age should not be considered a contraindication to esophagectomy. Most of these studies included a small number of elderly patients, and many studies only evaluated patients who underwent surgical resection. In addition, although the physiologic changes of getting older are significantly different between elderly patients and younger patients (12), there are few established standardized treatment strategies for elderly patients with EC (13). Thus, it is essential to identify these characteristics in elderly patients and to assess the effect of age on treatment decisions and outcomes.
Therefore, the national surveillance, epidemiology and end results (SEER) database spanning the years 1973 to 2013 was used to study the outcomes of elderly EC patients. As most of study on elderly EC stated the age threshold of 70 years used to define the elderly cohort (4-8,10), this study were to compare the clinical characteristics, treatment modality, outcomes and independent prognostic factors for EC in selected patients younger than 70 years of age to those in patients 70 years of age or older and to explore the role of resection or RT among the elderly.
Methods
Patients
The SEER database originates from 18 cancer registries covering almost 28% of the United States population and is sponsored by the National Cancer Institute. We analyzed the SEER Cancer Incidence Public Use Database (1973 to 2013), which was published in November 2013. EC cases (site codes, C15.0–C15.9) were extracted from the SEER database for the years 1973 to 2013. Exclusion criteria included cases without microscopic confirmation; diagnosis obtained at autopsy or by death certificate; no records of age, race, or sex; and lack of survival time while the patient is still alive.
A total of 61,799 patients with EC matching the specified criteria were included in the final analysis. Individual data retrieved for each case included age at diagnosis, gender, race, year of diagnosis, tumor histology, histological stage, treatment modality (RT/surgery), cause-specific death classification, vital status and months of survival. The entire patient population was divided into two age groups: patients less than 70 years of age and patients 70 years of age or older at diagnosis.
Statistical analysis
The chi-square test was used to evaluate the statistical significance of differences in the proportions of gender, race, tumor histology, histological stage, and treatment modality between the two age groups. Overall survival (OS) was defined as the time from medical diagnosis to death from any cause, and living patients were excluded at the time of last recording. Esophageal cancer-specific survival (ECSS) was defined as the time from medical diagnosis to death related to EC. OS and ECSS were estimated using the Kaplan-Meier method and compared using the log-rank test. Multivariate Cox proportional hazard regression was used to determine independent prognostic factors. The hazard ratio (HR) and corresponding 95% confidence interval (CI) were calculated. Statistical analyses were performed using SEER*stat and SPSS 20.0. The propensity score analysis (PSA) was performed using R software version 2.15.1 to remove confounding factors for matching the without any treatment and surgery or/and RT groups. Patients in the two groups were matched 1:1 using the nearest propensity score. After propensity score matching, OS and ECSS were re-evaluated. All statistical tests were two-sided, and P<0.05 was considered statistically significant.
Results
Demographics
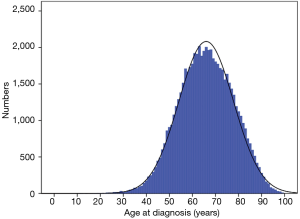
A total of 81,114 patients with EC were registered in 1973–2013, of which 61,799 (76%) met the entry criteria for this study. The median age of the included patients was 66 (95% CI, 65.88–66.13) years. The median [interquartile range (IQR)] follow-up time was 8 [3–18] months. Figure 1 shows the age distribution of all EC patients, and the peak age at incidence was between 60 and 80 years.
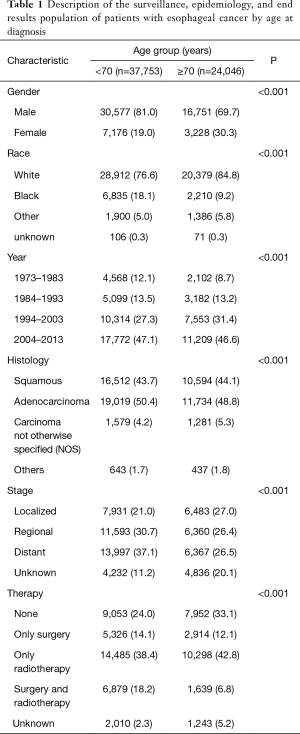
A total of 24,046 patients (38.9%) were 70 years of age or older, while 37,753 patients (61.1%) were younger than 70 years of age. The proportion of women with EC was significantly higher in the older group than in the younger group (30.3% vs. 19.0%; P<0.001). There was a higher proportion of White and American Indian or Asian/Pacific Islander patients but a lower proportion of Black patients in the older group than in the younger group (P<0.001). Additionally, there was a significantly higher proportion of elderly patients diagnosed in 1994–2003 and 2004–2013 but a lower proportion diagnosed in 1973–1983 and 1984–1993 (P<0.001) (Table 1).
Full table
Histology and staging
The distribution of tumor histology was considerably different between the two groups. Squamous cell histology was more frequent in the elderly age group: 44.1% in the older than 70 years of age group compared with 43.7% in the younger than 70 years of age group (P<0.001). Adenocarcinoma accounted for 50.4% of patients who were younger than 70 years of age and 48.8% of those older than 70 years of age (P<0.001). In addition, carcinoma NOS was more common in the older group, accounting for 5.3% and 4.2% of patients 70 years of age or older and patients younger than 70 years of age, respectively.
The staging distribution was also significantly different between age groups. The older group showed a higher proportion of localized disease than the younger group, whereas the younger group showed a higher proportion of distant and regional disease. All of the data are shown in Table 1 (P<0.001).
Therapy
The proportion of patients undergoing surgery (only surgery/surgery and RT) was significantly higher in younger patients (14.1%/18.2%) than in older patients (12.1%/6.8%); moreover, there were more patients had not any therapy in older group (33.1%) compared with younger group (24.0%).
Survival
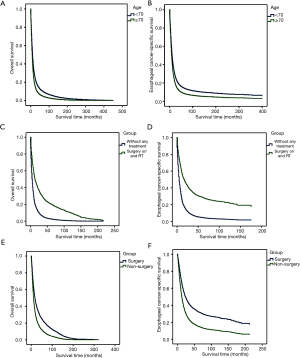
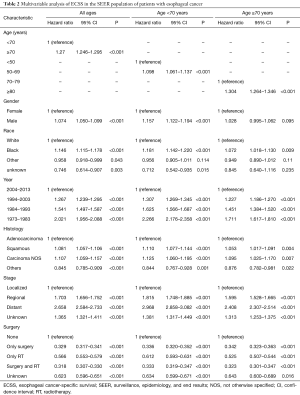
The OS (HR, 1.324; 95% CI, 1.300–1.348; P<0.001) and ECSS (HR, 1.270; 95% CI, 1.246–1.295; P<0.001) were lower for older patients than for younger patients (Figure 2A,B). We performed a multivariate analysis to control for the effect of gender, race, year of diagnosis, histology, tumor stage, and treatment modality on ECSS. The results of the multivariate analysis indicated that the risk of mortality from EC increased in patients 70 years of age or older relative to patients younger than 70 years of age (HR, 1.270; 95% CI, 1.246–1.295; P<0.001). The independent HR of death was highest for patients with distant-stage disease (HR, 2.658; 95% CI, 2.584–2.733; P<0.001). Black race (HR, 1.146; 95% CI, 1.115–1.178; P<0.001), male gender (HR, 1.074; 95% CI, 1.050–1.099; P<0.001), squamous cell (HR, 1.081; 95% CI, 1.057–1.106; P<0.001), carcinoma NOS (HR, 1.107; 95% CI, 1.059–1.157; P<0.001), and an earlier year of diagnosis (P<0.001) were also identified as significant independent negative prognostic factors. Those who had surgery or/and RT (P<0.001) were significant independent favorable prognostic factors (Table 2).
Full table
Comparison of prognostic factors between age groups
Univariate and multivariable analyses of ECSS in patients younger than 70 years of age and patients 70 years of age or older with EC were also performed. In the younger than 70 years of age group, factors independently associated with decreased ECSS on multivariable analysis included older age (HR, 1.098; 95% CI, 1.061–1.137; P<0.001), male sex (HR, 1.157; 95% CI, 1.122–1.194; P<0.001), black race (HR, 1.181; 95% CI, 1.142–1.220; P<0.001), earlier year of diagnosis (P<0.001), squamous cell (HR, 1.110; 95% CI, 1.077–1.144; P<0.001), and carcinoma NOS (HR, 1.125; 95% CI, 1.060–1.195; P<0.001). Receiving surgery or/and RT were independent favorable prognostic factors (Table 2). In addition, in the 70 years of age or older group, the multivariate analysis indicated that older age (HR, 1.304; 95% CI, 1.264–1.346; P<0.001), black race (HR, 1.072; 95% CI, 1.018–1.130; P=0.009), earlier year of diagnosis (P<0.001), squamous cell (HR, 1.053; 95% CI, 1.017–1.091; P=0.004), and carcinoma NOS (HR, 1.095; 95% CI, 1.025–1.170; P=0.007) were independent negative prognostic factors. Notably, receiving only surgical therapy (HR, 0.342; 95% CI, 0.323–0.363; P<0.001), both surgery and radiotherapy (HR, 0.323; 95% CI, 0.301–0.347; P<0.001) and only radiation therapy (HR, 0.525; 95% CI, 0.507–0.544; P<0.001) were independent favorable prognostic factors (Table 2).
PSA
The selection criteria for the PSA included patients 70 years or older with localized and regional disease; patients who had no records of surgery were excluded. Approximately 12,169 EC patients 70 years of age or older with localized and regional disease were divided into two groups: a without any treatment group (N=2,527, 20.8%), and a surgery or/and RT group (N=9,642, 79.2%). Each patient’s propensity score was calculated using a logistic regression model based on age, sex, race, year of diagnosis, tumor histology and historic stage. PSA created 2,465 pairs of patients. The results showed that OS (HR, 0.436; 95% CI, 0.409–0.464; P<0.001) and ECSS (HR, 0.406; 95% CI, 0.379–0.435; P<0.001) were significantly better in the surgery or/and RT group than in the without any treatment group (Figure 2C,D). Moreover, OS and ECSS at each year of diagnosis were also performed (Table S1). Trend of OS and ECSS showed increasing in surgery or/and RT group over three decades from 1984.
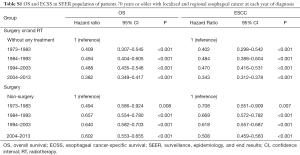
Full table
For the surgery or/and RT group of patients 70 years or older was also divided into two groups: the surgery group, and the non-surgery group. Then, 2,014 pairs of patients were created. The results showed that OS (HR, 0.649; 95% CI, 0.614–0.687; P<0.001) and ECSS (HR, 0.600; 95% CI, 0.563–0.639; P<0.001) were significantly better in the surgery group than in the non-surgery group (Figure 2E,F). Furthermore, trend of OS and ECSS also showed increasing in surgery group over three decades from 1984 (Table S1).
Discussion
The SEER database was used to thoroughly characterize and establish data regarding outcomes in elderly patient populations and compares them with the younger patient population. A multivariate analysis was also performed to identify the independent prognostic factors in patients younger than 70 years and in those 70 years or older. PSA regarding survival after different treatments were also performed in locoregional EC.
Our analysis confirms that a higher proportion of women have EC among older patients. Furthermore, female gender was an independent favorable prognostic factor for EC, which was similar to previously reported findings (14). However, the prognostic impact of sex differed between elderly patients and younger patients because gender was not an independent negative prognostic factor in patients 70 years of age or older. These findings may be explained by the hypothesis that the endocrine milieu in pre- and perimenopausal females functions as a protective factor against EC, while older postmenopausal females lose this estrogen exposure (15). In addition, males showed a higher incidence of drinking and smoking, which are also risk factors for inducing EC at an earlier age (1).
A higher proportion of patients 70 years of age or older were Caucasians, whereas a lower proportion were African Americans. This result may be related, in part, to racial differences in the prevalence of tobacco use and alcohol consumption, nutritional status patterns, and drinking beverages at high temperatures (16,17). In addition, black race was an independent adverse prognostic factor in all subgroups. A previous study reported that black patients had a higher risk of mortality than white patients because of their lower likelihood to undergo esophagectomy (18). Furthermore, this finding may also be related to the quality of care within certain health systems and race-related differences in patient-physician communication and socioeconomic status (19,20).
We also observed a trend for improved cancer outcome across all ages. The improved survival in the more recent time period may be a reflection of developments in therapy technology and early diagnosis or better supportive care measures. Neoadjuvant chemotherapy or chemo-RT was also more likely to be administered in the latter period and have been associated with improved survival (21).
Our study indicated that patients 70 years of age or older had a higher proportion of the histologic diagnosis of carcinoma NOS, suggesting that less invasive procedures may have been used in elderly patients. Therefore, sufficient tumor tissue may not have been available to make a precise sub-histologic diagnosis. Although the histology of squamous cell and adenocarcinoma was different between the two groups, the difference was too small to reach any important clinical significance. The statistically significant result was likely due to the large sample size.
We found that the older patient group showed a higher proportion of localized stage disease and a lower proportion of regional and distant-stage disease than the younger patient group. Several investigators have reported a lower occurrence of later-stage disease in older patients than in younger patients due to early diagnosis with a more thorough medical examination, whereas younger patients may delay diagnosis due to a lower suspicion of cancer (22,23). In addition, this difference may be associated with different genetic polymorphisms. For instance, one study showed that the MBD4 rs3138355 G>A polymorphism was associated with a significantly decreased risk of EC in elderly patients (24).
Most importantly, we observed a higher rate of without any treatment in older patients, whereas 33.1% of the elderly population comparing with only 24.0% of younger patients. Clearly, older patients were less likely to receive surgery or RT, which is similar to a report of regional cancer registry data in which only 23% of patients older than 85 years of age underwent surgery, compared to 55% of patients 65 to 70 years old (25). Our analysis showed that elderly patients were less likely to receive surgical therapy than younger patients despite a higher proportion of early stage disease in older groups. Frequent causes for the avoidance of surgery in these elderly patients, who have higher comorbidities, include the fear of increased operative adverse events and mortality.
A previous population-based analysis revealed that the outcomes in elderly patients after high-risk cancer surgery were considerably worse than those in younger patients because of the higher incidence of comorbidities and operative mortality (26). However, in a previous study, it was reported that no surgical therapy and no RT were independent negative prognostic factors for patients with EC (14). Furthermore, our data additionally indicated that surgery or/and RT were independent favorable prognostic factors in the 70 years of age or older group. This result indicated that elderly patients can benefit from appropriate surgery and/or RT and likely share similar benefits with younger patients. In addition, the propensity score matching analysis suggested that OS and ECSS were significantly better in the surgery or/and RT group of elderly patients with localized and regional disease. Therefore, the increased possibility of selecting less aggressive forms of therapy because of fear of increased adverse effects in elderly patients with greater comorbidities may contribute to the poor survival outcomes observed for this group. A study reported by Steyerberg et al. (25) indicated that elderly patients undergo less intensive treatment for EC, which is explained by both a lower rate of visiting a cancer expert and by less intensive treatment once seen. In addition, elderly patients 75 years of age or older, especially octogenarians, showed a relatively inferior prognosis compared with that of younger patients, partially because they received neoadjuvant therapy less often (13). Furthermore, another study showed that esophagectomy may be performed safely in carefully selected octogenarians, as these patients showed similar hospital mortality and no significant differences in cancer-related survival compared with younger patients (27). In addition, we found that survival benefits of surgery/RT increased gradually over time, which might be contributed to the improving techniques and supportive treatments. These findings evidenced the benefit and necessity of aggressive cancer reduction treatment among elderly EC patients. Further intentional studies are warranted.
Our study had some limitations. First, its retrospective nature may have resulted in some inevitable biases, as the SEER database does have not sufficient information to control for potentially confounding variables, such as comorbidities, performance status, treatment-related adverse effects, chemotherapy, nutritional status, tobacco use and alcohol consumption. Second, the SEER database does not provide data on marital status, socioeconomic status and value judgments of individual patients, which might have an impact on their choices of therapy. Furthermore, the time span covered in this study was greater than 30 years, and anesthesia, surgery, chemotherapy and postoperative care have significantly improved over these years. Despite these limitations, studies performed using the SEER database provide unique opportunities to evaluate a large number of patients 70 years of age or older, as few large prospective studies are performed on elderly patients with EC.
In summary, patients 70 years of age or older account for 38.9% of all EC cases in the SEER database, and these patients show distinctive clinical characteristics and inferior survival outcomes compared to younger patients. In addition, patients 70 years of age or older are less likely to be subjected to surgery or/and RT despite a higher proportion of localized disease. Specifically, surgery or/and RT were independently associated with better ECSS for elderly patients, suggesting that surgery or/and RT may be favorable prognostic factors among older patients. Thus, more investigations are needed to devise better treatment strategies thus to further improve outcomes for elderly patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Cijs TM, Verhoef C, Steyerberg EW, et al. Outcome of esophagectomy for cancer in elderly patients. Ann Thorac Surg 2010;90:900-7. [Crossref] [PubMed]
- Yang HX, Ling L, Zhang X, et al. Outcome of elderly patients with oesophageal squamous cell carcinoma after surgery. Br J Surg 2010;97:862-7. [Crossref] [PubMed]
- Markar SR, Karthikesalingam A, Thrumurthy S, et al. Systematic review and pooled analysis assessing the association between elderly age and outcome following surgical resection of esophageal malignancy. Dis Esophagus 2013;26:250-62. [Crossref] [PubMed]
- Ruol A, Portale G, Zaninotto G, et al. Results of esophagectomy for esophageal cancer in elderly patients: age has little influence on outcome and survival. J Thorac Cardiovasc Surg 2007;133:1186-92. [Crossref] [PubMed]
- Kinugasa S, Tachibana M, Yoshimura H, et al. Esophageal resection in elderly esophageal carcinoma patients: improvement in postoperative complications. Ann Thorac Surg 2001;71:414-8. [Crossref] [PubMed]
- Markar SR, Low DE. Physiology, not chronology, dictates outcomes after esophagectomy for esophageal cancer: outcomes in patients 80 years and older. Ann Surg Oncol 2013;20:1020-6. [Crossref] [PubMed]
- Karl RC, Smith SK, Fabri PJ. Validity of major cancer operations in elderly patients. Ann Surg Oncol 1995;2:107-13. [Crossref] [PubMed]
- Morita M, Egashira A, Yoshida R, et al. Esophagectomy in patients 80 years of age and older with carcinoma of the thoracic esophagus. J Gastroenterol 2008;43:345-51. [Crossref] [PubMed]
- McLean AJ, Le CDG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev 2004;56:163-84. [Crossref] [PubMed]
- Miyata H, Yamasaki M, Makino T, et al. Clinical Outcome of Esophagectomy in Elderly Patients With and Without Neoadjuvant Therapy for Thoracic Esophageal Cancer. Ann Surg Oncol 2015;22 Suppl 3:S794-801. [Crossref] [PubMed]
- Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: a SEER database analysis. J Gastroenterol Hepatol 2016;31:1141-6. [Crossref] [PubMed]
- Mathieu LN, Kanarek NF, Tsai HL, et al. Age and sex differences in the incidence of esophageal adenocarcinoma: results from the Surveillance, Epidemiology, and End Results (SEER) Registry (1973-2008). Dis Esophagus 2014;27:757-63. [Crossref] [PubMed]
- Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer 2009;125:491-524. [Crossref] [PubMed]
- Rasool S. Esophageal cancer: associated factors with special reference to the Kashmir Valley. Tumori 2012;98:191-203. [PubMed]
- Revels SL, Morris AM, Reddy RM, et al. Racial disparities in esophageal cancer outcomes. Ann Surg Oncol 2013;20:1136-41. [Crossref] [PubMed]
- Manfredi C, Kaiser K, Matthews AK, et al. Are racial differences in patient-physician cancer communication and information explained by background, predisposing, and enabling factors. J Health Commun 2010;15:272-92. [Crossref] [PubMed]
- Morris AM, Rhoads KF, Stain SC, et al. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg 2010;211:105-13. [Crossref] [PubMed]
- Pasquali S, Yim G, Vohra RS, et al. Survival After Neoadjuvant and Adjuvant Treatments Compared to Surgery Alone for Resectable Esophageal Carcinoma: A Network Meta-analysis. Ann Surg 2017;265:481-91. [Crossref] [PubMed]
- Oezcelik A, Ayazi S, DeMeester SR, et al. Adenocarcinoma of the esophagus in the young. J Gastrointest Surg 2013;17:1032-5. [Crossref] [PubMed]
- Ramalingam S, Pawlish K, Gadgeel S, et al. Lung cancer in young patients: analysis of a Surveillance, Epidemiology, and End Results database. J Clin Oncol 1998;16:651-7. [Crossref] [PubMed]
- Lee JM, Shun CT, Wu MT, et al. The associations of p53 overexpression with p53 codon 72 genetic polymorphism in esophageal cancer. Mutat Res 2006;594:181-8. [Crossref] [PubMed]
- Steyerberg EW, Neville B, Weeks JC, et al. Referral patterns, treatment choices, and outcomes in locoregional esophageal cancer: a population-based analysis of elderly patients. J Clin Oncol 2007;25:2389-96. [Crossref] [PubMed]
- Finlayson E, Fan Z, Birkmeyer JD. Outcomes in octogenarians undergoing high-risk cancer operation: a national study. J Am Coll Surg 2007;205:729-34. [Crossref] [PubMed]
- Zehetner J, Lipham JC, Ayazi S, et al. Esophagectomy for cancer in octogenarians. Dis Esophagus 2010;23:666-9. [Crossref] [PubMed]