Two cases of combined thoracoscopy and open chest surgery for locally advanced lung carcinoma
Introduction
Although it has been reported that thoracoscopic surgery (TS) is less invasive than open chest surgery for lung cancer (1-3), it is still controversial in indication of locally advanced lung carcinoma (clinical stage T3–4). In our hospital, we perform TS by creating four 5–20-mm access ports. Thoracoscopy can provide a magnified view via an optical camera and can explore blind spots that cannot be directly viewed; therefore, it is useful for operations of deep parts of the thoracic cavity. We believe that adapting the procedure by combining thoracoscopy and open chest surgery is possible, with the aim of reducing the invasiveness of surgery for advanced lung carcinoma while ensuring that the operation is safe and curative.
Case presentation
Case 1
A man in his 60s had an abnormal shadowing on a chest X-ray during a medical checkup. Computed tomography (CT) revealed a 40-mm-sized tumor that was suspected to have infiltrated into the chest wall and paraaortic and hilar lymph nodes (Figure 1A). Pathological examination of tumor samples obtained by CT-guided biopsy led to a diagnosis of primary lung adenocarcinoma. Tumor possibly involved the parietal and mediastinal pleura. Lymph node metastasis on N2 region was strongly suspected. Primary clinical stage using CT scan, PET-CT, and brain MRI was cT2a-T3N2M0, stage IIIA (UICC 7th edition). Bronchoscopy was not performed because tumor specimen was gotten by the needle biopsy, and lymph node metastasis was evaluated on CT. Then induction therapy was selected. After completing two courses of cisplatin plus vinorelbine and 40 Gy radiotherapy, clinical stage remained IIIA (ycT2aN2M0).
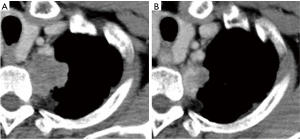
However, a slight space appeared between the main tumor and the chest wall/subclavian artery on CT. We considered that the tumor could be separated from the mediastinum on the parietal pleura at the periostal level (Figure 1B). Furthermore, FDG accumulation on PET-CT of lymph node regressed. A curative resection was then decided to be practicable.
The radiological findings revealed that combined resection of the chest wall or blood vessels was unnecessary. The lymph nodes alsodecreased in size, although their detachment from the pulmonary artery was expected to be difficult due to adhesions owing to inflammation.
Because the view in open chest surgery has many blind spots in the deeper parts of the thoracic cavity, there is a risk for vascular or nerve damage while separating the adhesions. Thus, we considered using the hook approach or an approach via a midline thoracotomy, such as the trans manubrial approach and hemi-clamshell approach (4), which would entail a large chest incision. As thoracoscopy enables these blind spots to be explored and offers a magnified view, we considered that this would be useful for work in deeper parts if costectomy or angioplasty were not required.
The operations in the thoracic apex were performed first via TS, with the patient in the right decubitus position under general anesthesia. Because of chemoradiotherapy, the tumor invasion site in the apical region firmly adhered to the chest wall. The surfaces of the subclavian artery and left brachiocephalic vein were exposed, and a wide area of mediastinal pleura was removed. The thoracoscopic view in the apical region was extremely good, and the boundary between blood vessels and lung surface was clearly visualized (Figure 2A,B). Bleeding while separating the adhesion was appropriately dealt with, enabling operations to be conducted blood free. Although manual exploration could not be done under thoracoscopy, pathological examination using frozen section was useful. The frozen section showed the absence of any residual tumor in the cut end of the chest wall during the operation.
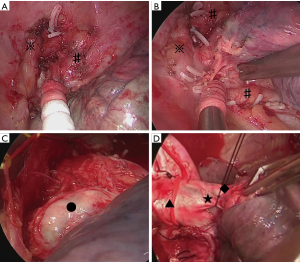
A lateral incision that measured approximately 10 cm was then performed in the 5th intercostal space. After the shift to open surgery, the pulmonary artery trunk was identified within the pericardium, and the blood vessels were dealt with carefully. Because the phrenic, vagus, and recurrent nerves were exposed during thoracoscopy, safely and easily performing lymph node dissection using small thoracotomy was possible (Figure 2C,D). The operation took 328 minutes, and 60 mL blood was lost.
The postoperative course was uneventful, and the patient was discharged from hospital on postoperative day 4. Because a pathological complete response (ypT0N0M0, stage 0) was achieved, the patient remains under observation without any additional treatment. He continues to be recurrence free after 1.5 years.
Case 2
A non-smoking woman in her 70s was treated for left-sided pneumonia several years before because she was followed up for persistent shadows on her X-rays. During this time, she repeatedly developed pneumonia, and the persistent shadows enlarged. Thus, transbronchial lung biopsy was performed, and she was diagnosed with primary lung adenocarcinoma.
Chest CT revealed a mass measuring approximately 5 cm in size in the left apical region that was in contact with the mediastinal side of the apex. Consolidation around the tumor also extended into the hilar region (Figure 3). It was considered that there was no invasion into the subclavian artery, left brachiocephalic vein, or chest wall in the apical region. However, it was anticipated that strong inflammatory adhesions would be present because the patient repeatedly had pneumonia for the past several years. The clinical staging was considered to be stage IB–IIB (cT2a-T3N0M0). Hence, we decided to perform the surgery.
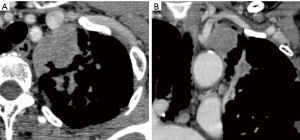
The superior margin was in contact with the left brachiocephalic vein and left subclavian artery, and the inferior margin spread along the mediastinum as far as the central pulmonary artery. Therefore, an approach via a median sternotomy was selected. However, when only a median sternotomy is performed, visualizing the areas of the dorsal hilar region, interlobular region, and area around the recurrent nerve may be difficult. To avoid such a limited view, we considered using the hemi-clamshell approach. So, in this case, the thoracoscopic procedure would be useful to operate the blind region with the median view.
The thoracoscopic part was performed first, with the patient in the right decubitus position under general anesthesia. The procedures around the vagus nerve in the dorsal hilar region, interlobular separation, work with the pulmonary artery in the interlobular region to the lingual segment, and dissection of the caudal end of the interlobular lymph nodes were conducted using a thoracoscope (Figure 4A,B).
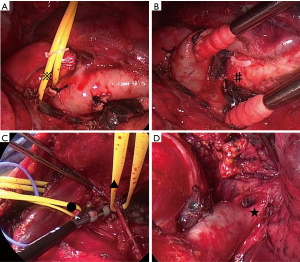
As expected from the preoperative assessment, the tumor strongly adhered to the left brachiocephalic vein. Actually, it was difficult to see the apical and median region with lateral view when we were operating under thoracotomy. To avoid the risk for bleeding at the apical area, the patient was shifted to the supine position, and the surgery via median sternotomy was initiated. During open surgery, the left brachiocephalic vein was identified and carefully separated from the tumor. The adhesions to the mediastinum were also separated while carefully preserving the phrenic nerve. Next, the superior pulmonary vein, branches of the central pulmonary artery (A3, A1+2a+b), and upper bronchus were all cut off. Dissection of the mediastinal lymph nodes was also performed (Figure 4C,D). The operation took 261 minutes, and a total of 200 mL blood was lost.
There were no postoperative complications, and the patient was discharged from hospital on postoperative day 8. Pathological findings showed that complete resection was achieved, with no pleural invasion identified. Although the diagnosis was stage IIIA primary lung adenocarcinoma [pT2aN2 (subaortic lymph node metastasis) M0], because of the patient’s age, adjuvant chemotherapy was not performed and she was kept under observation. She continues to be recurrence free after 3 years.
Discussion
TS has recently become the standard treatment for early stage lung carcinoma, and some studies have also described its value in terms of reducing invasiveness (1-3). However, the indications for its usefulness in cases of advanced lung carcinoma are unclear. In particular, it remains controversial whether TS should be indicated for curative surgery when adjacent organs such as blood vessels and chest wall must also be resected or any of these structures need to be reconstructed.
In case 1, separating the chest wall and subclavian artery for post-chemoradiotherapy adhesions was the key point for complete resection. Combining thoracoscopy and open surgery enabled this detachment to be reliably performed. In case 2, widespread adhesions were anticipated because of repeated pneumonia. Although median sternotomy was required to conduct the detachment while preserving the left brachiocephalic vein and phrenic nerve, thoracoscopy provided cover for the dorsal hilar region, interlobar region, and surroundings of the recurrent nerve, which are often blind spots during a median sternotomy. In both cases, performing the thoracoscopy first was including the role of exploration in the thoracic cavity. If cancerous infiltration was strongly suspected by the thoracoscopy, we would have performed an open surgery.
Lorenzo et al. previously reported regarding combining thoracoscopy and thoracotomy in a case of a pancoast tumor, as well as six other case reports. They also found that this was useful for apical operations in addition to reducing surgical invasiveness (5).
However, caution is necessary while dealing with the pulmonary artery when there is inflammation or tumor infiltration in the lymph nodes around the central pulmonary artery. Previous reports have mentioned that the fixation of lymph nodes and a vascular injury are the most frequent causes for converting thoracoscopic procedure to open surgery (6,7). At this point, if the effect of preoperative treatment for hilar lymph node metastasis or inflammatory changes is anticipated, thoracotomy may be better than thoracoscopy where any unexpected situations may occur because they can be rapidly dealt with using a wide range of treatments. In particular, the bleeding from injuring A3, the first branch of the left pulmonary artery, can be fatal. So, the surgical approach should be carefully selected.
In addition, combining the thoracoscopy with open surgery, total invasiveness as well as factors such as postoperative pain, wound infection, and time of recovery are reduced (5). In fact, in this study, case 1 did not require the epidural catheter and the chest drainage tube was also removed. He was ambulant around the ward in the evening of operative day. Cerfolio introduced his new technique for minimally invasive chest wall resection. If we could use this technique, the indication of combined surgery could be further expanded, such as for the case of T3/T4 tumors (8).
We are certain that hilar operations can be safely performed using a thoracoscope even in patients who underwent induction chemotherapy; however, if the risk of bleeding is expected in case of emergency, open procedure is a safe method. The open approach is needed in the following cases: High risk of bleeding; for example, it is expected the adhesions between the tumor and great vessels, and hilar lymph node and pulmonary artery. The lack of curability; it need to resect the surround organs, such as chest wall, intrathoracic vessels, diaphragm, and atypical procedures. Thoracoscopy is also useful for confirming the state of the tumor spread at any sites such as the apex of the thoracic cavity and the diaphragm surface where the field of view is obscured during thoracotomy.
In patients who require local procedures in both the hilar region and separately in the chest wall or diaphragm, combination surgery we reported in this study can be used to perform one or other tasks, thereby possibly reducing the invasiveness of surgery while ensuring its safety and curative effect. We have to admit that thoracoscopic surgery for T3/T4 tumors remains controversial. However, we believe that rather than rigidly adhering to a specific procedure, surgeons must become proficient in a range of different methods, utilizing these to their best ability while considering both oncological outcomes and patient safety.
Conclusions
TS can be used as curative surgery for early stage lung carcinoma and for treating locally advanced lung carcinoma. In response to the increasing complexity of thoracic surgery, surgical procedures must be adapted in various ways. Having a thorough understanding of the features of TS and open chest surgery and integrating the two methods may enable surgical procedures that are guaranteed to be curative, safe, and minimally invasive.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Each patient was informed that his clinical data could be used for various clinical studies, and written informed consent for this report was obtained on this basis.
References
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Lobectomy--video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg 1995;109:997-1001; discussion 1001-2. [Crossref] [PubMed]
- Cai YX, Fu XN, Xu QZ, et al. Thoracoscopic lobectomy versus open lobectomy in stage I non-small-cell lung cancer: a meta-analysis. PLoS One 2013;8:e82366. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. ESTS Database Committee and ESTS Minimally Invasive Interest Group. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Shintani Y, Kanzaki R, Kawamura T, et al. Surgical resection for advancesd lung cancer using the hemi-clamshell approach. Interact Cardiovasc Thorac Surg 2017;25:462-8. [Crossref] [PubMed]
- Rosso L, Palleschi A, Mendogni P, et al. Video-assisted pulmonary lobectomy combined with transmanubrial approach for anterior Pancoast tumor resection: case report. J Cardiothorac surg 2016;11:65. [Crossref] [PubMed]
- Puri V, Patel A, Majumder K, et al. Intraoperative conversion from video-assisted thoracoscopic surgery lobectomy to open thoracotomy: a study of causes and implications. J Thorac Cardiovasc Surg 2015;149:55-61,62.e1.
- Byun CS, Lee S, Kim DJ, et al. Analysis of unexpected conversion to thoracotomy during thoracoscopic lobectomy in lung cancer. Ann Thorac Surg 2015;100:968-73. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Minimally invasive chest wall resection: sparing the overlying, uninvolved extrathoracic musculature of the chest. Ann Thorac Surg 2012;94:1744-7. [Crossref] [PubMed]


