Lymph node metastases near the celiac trunk should be considered separately from other nodal metastases in patients with cancer of the esophagus or gastroesophageal junction after neoadjuvant treatment and surgery
Introduction
Cancer of the esophagus and gastroesophageal junction (GEJ) is an aggressive disease and notorious for rapid dissemination (1). The preferred curative strategy for patients with esophageal or GEJ cancer without distant metastases consists of neoadjuvant chemo(radio)therapy followed by esophagectomy (2,3).
In the seventh edition of the American Joint Committee on Cancer (AJCC) esophageal TNM staging system, the number of node metastases plays a pivotal role (4). The N stage is subdivided into N0–N3 based on the number of involved nodes. However, the present staging system does not take into account the location of nodal metastases. In former TNM staging systems, positive lymph nodes near the celiac axis were seen as distant metastatic (M1a) disease (4).
Truncal lymph nodes lie around the celiac artery, which is 1–2 cm long and trifurcates into the left gastric, common hepatic and splenic artery (5). There is considerable controversy surrounding the clinical importance of lymph node metastases near the celiac trunk in patients with cancer of the esophagus or GEJ (5-7). This has several reasons. In both the diagnostic process and the pathologic specimen interpretation, it may be hard to differentiate between celiac nodes, and nodes around the hepatic, splenic and left gastric artery (5) as they are anatomically very close to one another.
Surgeons historically have given patients with small resectable lymph node metastases near the celiac trunk the “benefit of the doubt” and have proceeded with resection (6). Although long-term survival was achieved in a small percentage of patients, these nodes were considered as an indicator of poor prognosis (6). However, the eradication of lymphadenopathy with neoadjuvant therapy is possible and therefore, it has been suggested that these patients were good candidates for neoadjuvant treatment regimens followed by surgery (5).
In the neoadjuvant era, it is unclear what the clinical relevance of these lymph nodes is, whether they can be identified preoperatively and what the prognostic value of these nodes is. Therefore the aim of the present study is to identify the incidence and prognostic significance of celiac trunk metastasis in patients who underwent neoadjuvant chemo(radio) therapy followed by a esophagectomy.
Methods
Patient population
Between March 1994 and September 2013 all consecutive patients with cancer of the mid-to-lower thoracic esophagus or GEJ who underwent potentially curative esophageal resection after neoadjuvant chemo(radio)therapy were included in the present study. Patients were selected from a prospectively collected and maintained database at the Department of Surgery, Academic Medical Centre, Amsterdam, the Netherlands. Patients with histologically confirmed, potentially curable squamous-cell carcinoma, adenocarcinoma, or large-cell undifferentiated carcinoma of the esophagus or GEJ were eligible for inclusion in the study. The study was approved by the institutional ethics committee of the Academic Medical Centre, Amsterdam, the Netherlands.
Pretreatment staging
Initial staging consisted of endoscopy with biopsy, endoscopic ultrasonography (EUS), external ultrasonography of the neck and a thoracoabdominal CT scan. A PET (CT) scan was not part of the standard initial staging, but was performed in some cases as part of a research protocol, by the referring physicians and in more recent years, after neoadjuvant CRT.
Pretreatment staging of truncal nodes
The diagnostic criteria for malignant lymph node involvement were size 1 cm or larger; round shape; homogeneous hypoechoic pattern; and sharp and distinct borders. Suspected lymph nodes in the region of the celiac trunk were defined as such when they were located within 2 cm of the celiac trunk on EUS or (PET-) CT. These nodes were not considered a contraindication for resection when patients were otherwise resectable. Patients with distant metastases did not undergo curative treatment and were not included in this study.
Neoadjuvant therapy
Neoadjuvant chemoradiotherapy (CRT) followed by esophagectomy was indicated in patients deemed fit for surgery with histologically proven, locally advanced, resectable esophageal malignancy without distant metastases (cT1N+M0 or cT2-4aN0-3M0).Two neoadjuvant CRT regimens were employed in the present study. Most patients received 23 fractions of 1.8 Gy (41.4 Gy) external-beam radiotherapy combined with weekly administered carboplatin (AUC2) and paclitaxel (50 mg/m2). Additionally, as part of a phase II clinical trial, a small proportion of patients received panitumumab (human monoclonal antibody to the epidermal growth factor receptor), at a dose of 6 mg/kg in addition to the standard neoadjuvant CRT (ClinicalTrials.gov Identifier: NCT01077999). Also, some patients were treated with CRT combined with deep loco-regional hyperthermia as part of a clinical trial (8). Radiation fields included the primary tumor plus a margin of at least 3 cm in caudal direction, plus all clinically suspect lymph nodes. Whether the truncal lymph nodes were included in the radiation fields was not recorded.
Neoadjuvant chemotherapy [EOX (epirubicin, oxaliplatin, capecitabine) or ECC (epirubicin, cisplatin, capecitabine)] was administered when the tumor bulk was located in the gastric cardia with involvement of the lower-thoracic esophagus (4).
Patients were restaged after neoadjuvant chemo(radio)therapy with CT, PET or PET-CT. Patients who developed distant metastases during neoadjuvant treatment were not operated. Also, patients with unresectable tumors during exploratory surgery or macroscopically incomplete resections (R2) and patients who died in hospital after surgery, were not included in the present study.
Surgery
Esophagectomy was performed by means of an open or minimally invasive transthoracic or transhiatal approach, as previously described by our institute (9-11). Surgery was performed within 6–10 weeks after completion of neoadjuvant chemo(radio)therapy.
Pathology
Pathologic findings were described in a standardized format by an experienced gastro-intestinal pathologist. In the absence of macroscopic tumor, any abnormal-appearing tissue was paraffin-embedded in total in order to make an adequate assessment for the presence of residual tumor and the effects of therapy. When no tumor cells were seen in the proximal, distal and circumferential resection margins, the resection was classified as R0. To grade the response to neoadjuvant CRT, the degree of histomorphologic regression was classified using the Mandard score (12). Pathologic complete response was defined as absence of viable tumor in the surgical resection specimen (both the esophagus and resected lymph nodes).
The origin of the left gastric artery and the subcarinal nodes were marked in the resection specimen by the surgeon. Separate lymph nodes (e.g., paratracheal) were marked by location and analyzed separately. Larger lymph nodes were cut in two and routine H&E staining was performed using a standardized protocol.
Definition of positive nodes near the celiac trunk
Lymph nodes near the origin of the left gastric artery were always removed as part of an esophagectomy. The origin of the left gastric artery was routinely marked by the surgeon. Nodes within 1 cm of this location in the specimen were considered truncal nodes in the present study. Furthermore, separate positive lymph nodes found at the hepatic artery and splenic artery were also classified as truncal nodes.
Follow-up
During the first 5 years after surgery all patients were seen at the outpatient clinic at three to four month intervals during the first 2 years and every 6 months during the next 3 years. After 5 years, follow-up data was obtained by telephone from the patient or the patient’s family practitioner. Recurrent disease was diagnosed on clinical grounds. When recurrence was suspected, additional investigations were performed.
Statistics
Statistical calculations were performed by SPSS software, version 20.0 (SPSS, Chicago, IL, USA). Differences between groups were tested by the Mann-Whitney U-test for continuous data. To compare categorical data, the Chi-square or Fisher exact test was used. Multivariate Cox regression analysis was carried out to identify independent prognostic factors. All factors from the univariate analysis with a P value less than 0.05 were entered in this multivariate analysis. P values less than 0.05 (two-sided) were considered statistically significant.
Results
Between March 1994 and September 2013, 503 patients with cancer of the mid-to-lower thoracic esophagus or GEJ underwent esophageal resection after neoadjuvant chemo(radio)therapy. In 6 (1.2%) patients metastases detected during surgical exploration lead to a palliative resection. Fourteen (2.8%) patients underwent a salvage resection (long course CRT, or residual disease after definitive CRT). Both groups were excluded. Twenty-one (4.2%) died due to postoperative complications. These patients were excluded.
Of the 462 included patients 345 were male (74.7%). The tumor was located in the mid-thoracic esophagus in 87 (18.8%), in the lower thoracic esophagus in 283 (61.3%) and at the GEJ/cardia in 92 patients (19.9%). The majority (68.6%) had an adenocarcinoma, with the following clinical staging (Table 1).
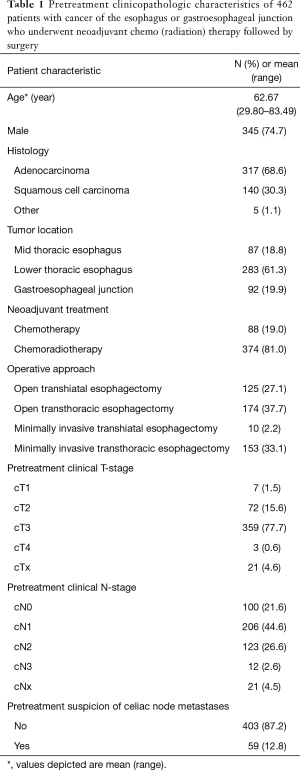
Full table
Eighty-eight patients (19.0%) underwent preoperative chemotherapy and 374 (81.0%) underwent neoadjuvant CRT (Table S1). Different types (open and minimal invasive) of transthoracic and transhiatal surgery were performed in the inclusion period (Table 1). A total of 439 (95.0%) patients underwent a radical (R0) resection; microscopic tumor was left behind (R1 resection) in the other 23 patients (5.0%) (Table 2).

Full table

Full table
Positive nodes near the celiac trunk
According to the marked pathology specimen, in 71 (15.4%) patients metastases were localized in the truncal nodes. Metastases in truncal nodes occurred significantly more frequent in male patients with adenocarcinoma and in patients with tumors located at the GEJ (Table 3). Pretreatment stage was significantly more advanced for both tumor depth (cT stage) and number of suspected lymph nodes (cN stage).
Postoperative characteristics of aggressive disease (Table 3) were significantly related with celiac node metastases (worse Mandard score, higher ypT and ypN stage and a poor differentiation).
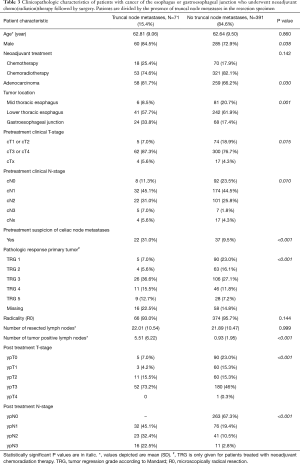
Full table
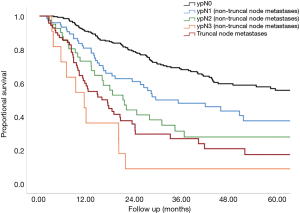
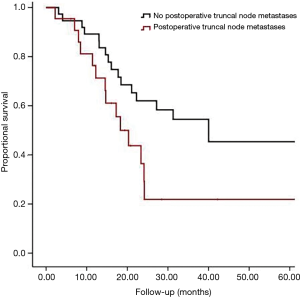
The median survival in patients with postoperative positive truncal nodes was 17 months (95% confidence interval, 11 to 24 months) in comparison to 55 months, (95% confidence interval, 37 to 72 months) for patients without truncal metastasis In Figure 1, the patients without truncal node metastases are divided into four groups: ypN0 (n=263, median survival 82 months), ypN1 (n=76, median survival 35 months), ypN2 (n=41, median survival 21 months) and ypN3 (n=11, median survival 11 months).
In multivariate analysis ypN stage, ypT stage, pathological response as well as positive truncal nodes were independently associated with survival (Table 4).
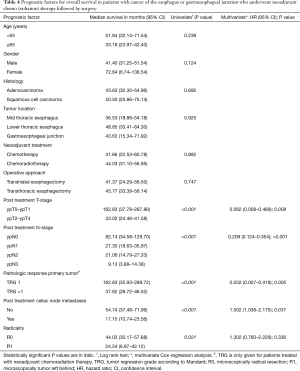
Full table
Preoperative suspected nodes near the celiac trunk
In 59 (12.8%) patients there was suspicion on metastases localized in truncal nodes based on EUS and (PET-) CT findings before the start of neoadjuvant therapy. Although there was a significant increase in the chance of having truncal node metastases when pretreatment evaluation showed suspicious truncal nodes, only 22 (31.0%) of 71 patients with positive truncal nodes were identified preoperatively with EUS or (PET-)CT. Interestingly, in 37 patients (62.7%) suspicious truncal nodes were identified preoperatively and may have been included in the radiation fields, but in the pathology specimen truncal node metastases were not found. These 37 patients without truncal node metastases in the resection specimen had a median overall survival of 40 months (95% confidence interval, 7 to 73 months) (Figure 2). Patients with truncal node metastasis had significantly (P=0.021) more often hematogenous dissemination but not peritoneal/pleural and locoregional lymphatic metastases.
Discussion
Lymphatic dissemination remains the most important predictor for survival in patients with cancer of the esophagus or GEJ. In the present study it has been demonstrated that not only the number of involved nodes but also the presence of positive truncal nodes in the surgical specimen after neoadjuvant treatment harbors important prognostic information. Thus, celiac nodal involvement is a strong predictor for widespread microscopic disease that may become evident later. The TNM-staging system, based on depth of tumor invasion, number of positive lymph nodes and distant metastases (4,13-15), is the most frequently used system to predict prognosis. This staging system is based on patients treated with primary surgery. However, this staging system is also applied to patients who are treated with neoadjuvant therapy and have complete, partial or no response to chemo(radiation) therapy in their primary tumor and lymph nodes. The present study shows that patients with positive truncal nodes after chemo(radiation) therapy have a dismal prognosis. In former TNM staging systems, positive lymph nodes near the celiac axis were categorized as M1a disease. Using this previous TNM staging system Schomas et al. assessed the impact of truncal node metastases in 310 patients who underwent esophagectomy between 1976 and 1999 for carcinoma of the distal esophagus or gastro-esophageal junction (16). The 52 patients (17%) with celiac node involvement had a significantly worse survival compared to pN0 patients, but no significant difference in survival was found in the comparison with pN+ patients without truncal node metastases. The authors concluded that the number of positive nodes, not their location, correlated best with survival (16). Studies like this led to the mentioned adaption in the latest TNM staging system in which the number of involved lymph nodes is the sole determining factor of the nodal status (4). However, the present study suggests that the presence of positive truncal nodes has important prognostic implications. It should be documented in the standard pathology report. The prognostic value of these nodes should be confirmed in larger multi-center series, but the inclusion of truncal nodes should be considered as a subcategory in future TNM staging systems.
In almost 63% of patients with suspicious truncal nodes before neoadjuvant therapy, truncal node metastases were not present in the resection specimen, and in one third of patients not even a single node was positive. These results suggest that the eradication of lymphadenopathy (also of truncal nodes) with neoadjuvant therapy is possible and predicts good survival. However, as described before (17), it is also possible that pre-treatment screening is not very specific for assessing truncal nodes. Suspicious pre-treatment node metastases were not proven with EUS-FNA in the present study, since positivity of truncal nodes was not a contraindication for surgery. Based on the study by Grotenhuis et al., the accuracy of CT imaging alone in detecting truncal node metastases is merely 70% (17). Furthermore, it should be realized that techniques for disease staging improved considerably during the time span of the study. The length of this study period [1994–2013] has also caused a certain heterogeneity in terms of treatment characteristics. A variation of both chemotherapy and CRT regimes was used. Despite these differences the type of neoadjuvant therapy was not significantly associated with overall survival on univariate analysis. This finding is consistent with that of previous trials comparing perioperative chemotherapy and preoperative CRT for esophageal carcinoma where no significant differences were demonstrated in terms of lymph node yield or survival (18). The operative approach (transhiatal versus transthoracic) did not seem to influence overall survival in our univariate analysis and based on recent literature we have no reason to expect that the switch towards minimally invasive surgery has had an effect on lymph node yield or survival (11,19). Another limitation is the lack of information on whether the truncal lymph nodes were within the radiation field. The question whether positivity of truncal lymph nodes was a failure of the pretreatment or just not treated (outside radiation field) cannot be answered. Consequently the question whether inclusion of positive truncal lymph nodes in the radiation field could have influenced the survival cannot be answered. Nevertheless, the low rate of positive truncal lymph nodes in the surgical specimen in patients with suspect truncal nodes at pre-staging does suggest an effect of preoperative treatment. Therefore, patients should not be declined neoadjuvant therapy followed by surgery based on pre-treatment characteristics of suspicious truncal nodes (20). Another limitation of the present study, and actually previous studies as well (5), is the difficulty to distinguish—both preoperatively and in the resection specimen—nodes along the left gastric artery from nodes near the celiac axis itself. Distance to the celiac trunk is hard to measure and it is not clear yet within which distance to the celiac trunk a node should be considered as a truncal node (5). Furthermore, in the present study also lymph nodes near the hepatic artery and splenic artery were considered as positive truncal nodes. However, these nodes were not separately marked. Assessing the nodal stations separately, might improve the adequacy of staging. For adenocarcinoma there are only few studies that describe the exact localization of mediastinal and abdominal lymph node metastases (21-23). Castoro et al. describe the localization of lymph node metastases in relation to neoadjuvant therapy and found no difference in celiac axis positive nodes between patients treated with surgery alone, neoadjuvant chemotherapy or neoadjuvant CRT (21).
The dismal prognosis of patients with positive truncal nodes in the surgical resection specimen warrants further prospective studies. Future trials assessing the accuracy of EUS and EUS-guided FNA and PET-CT scanning before and after neoadjuvant therapy and the influence of radiation fields are needed. There may even be place for diagnostic laparoscopy to sample truncal nodes in selected patients after neoadjuvant therapy.
In our study it is shown that although patients with positive truncal nodes have a poor prognosis, their survival is still superior compared to patients treated with palliative chemotherapy for distant metastatic disease (24-27) and superior to patients treated with definitive CRT who developed celiac node failures (28). This observation suggests that the presence of truncal node metastases, both before and after neoadjuvant treatment, should not be an absolute contraindication for esophagectomy. However, when truncal node metastases are confirmed after neoadjuvant therapy, their impact on prognosis should be an explicit part of patient counselling.
At his moment there is no evidence to support the use of additional postoperative (chemo)therapy in patients with truncal node metastases, but the presence of these metastases should be a stratification factor in the upcoming trials on adjuvant therapy for esophageal cancer.
In summary, positive truncal nodes found in the resection specimen after neoadjuvant therapy identify a subgroup of patients with a dismal prognosis. It reflects a particularly aggressive disease behavior and should therefore be considered specifically during both preoperative and postoperative decision making.
Acknowledgements
Funding: SM Lagarde is supported by a Koningin Wilhelmina Fonds (KWF, Dutch Cancer Society) Fellowship, UVA 2013-5853.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethics committee of the Academic Medical Centre, Amsterdam, the Netherlands.
References
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Sobin LH, Compton CC. TNM seventh edition: What's new, what's changed. Cancer 2010;116:5336-9.
- Ferguson MK. Difficult Decisions in Thoracic Surgery. Springer, 2011.
- Hulscher JB, Buskens CJ, Bergman JJ, et al. Positive peritruncal nodes for esophageal carcinoma. Dig Surg 2001;18:98-101. [Crossref] [PubMed]
- Eloubeidi MA, Wallace MB, Hoffman BJ, et al. Predictors of survival for esophageal cancer patients with and without celiac axis lymphadenopathy: impact of staging endosonography. Ann Thorac Surg 2001;72:212-9. [Crossref] [PubMed]
- Albregts M, Hulshof M, Zum Vörde Sive Vörding P, et al. A feasibility study in oesophageal carcinoma using deep loco-regional hyperthermia combined with concurrent chemotherapy followed by surgery. Int J Hyperthermia 2004;20:647-59. [Crossref] [PubMed]
- Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000; discussion 1001. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [Crossref] [PubMed]
- Lagarde SM, Reitsma JB, de Castro SM, et al. Prognostic nomogram for patients undergoing oesophagectomy for adenocarcinoma of the oesophagus or gastro-oesophageal junction. Br J Surg 2007;94:1361-8. [Crossref] [PubMed]
- Lagarde SM, Reitsma JB, Ten Kate FJ, et al. Predicting individual survival after potentially curative esophagectomy for adenocarcinoma of the esophagus or gastroesophageal junction. Ann Surg 2008;248:1006-13. [Crossref] [PubMed]
- Lagarde SM, ten Kate FJ, Reitsma JB, et al. Prognostic factors in adenocarcinoma of the esophagus or gastroesophageal junction. J Clin Oncol 2006;24:4347-55. [Crossref] [PubMed]
- Schomas DA, Quevedo JF, Donahue JM, et al. The prognostic importance of pathologically involved celiac node metastases in node-positive patients with carcinoma of the distal esophagus or gastroesophageal junction: a surgical series from the Mayo Clinic. Dis Esophagus 2010;23:232-9. [Crossref] [PubMed]
- Grotenhuis BA, Wijnhoven BP, Poley JW, et al. Preoperative assessment of tumor location and station-specific lymph node status in patients with adenocarcinoma of the gastroesophageal junction. World J Surg 2013;37:147-55. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Anderegg MC, Gisbertz SS, van Berge Henegouwen MI. Minimally invasive surgery for oesophageal cancer. Best Pract Res Clin Gastroenterol 2014;28:41-52. [Crossref] [PubMed]
- Lee PC, Port JL, Paul S, et al. Predictors of long-term survival after resection of esophageal carcinoma with nonregional nodal metastases. Ann Thorac Surg 2009;88:186-92. [Crossref] [PubMed]
- Castoro C, Scarpa M, Cagol M, et al. Nodal metastasis from locally advanced esophageal cancer: how neoadjuvant therapy modifies their frequency and distribution. Ann Surg Oncol 2011;18:3743-54. [Crossref] [PubMed]
- van de Ven C, De Leyn P, Coosemans W, et al. Three-field lymphadenectomy and pattern of lymph node spread in T3 adenocarcinoma of the distal esophagus and the gastro-esophageal junction. Eur J Cardiothorac Surg 1999;15:769-73. [Crossref] [PubMed]
- Ruffato A, Mattioli S, Perrone O, et al. Esophagogastric Metaplasia Relates to Nodal Metastases in Adenocarcinoma of Esophagus and Cardia. Ann Thorac Surg 2013;95:1147-53. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Prithviraj GK, Baksh K, Fulp W, et al. Carboplatin and paclitaxel as first-line treatment of unresectable or metastatic esophageal or gastric cancer. Dis Esophagus 2015;28:782-7. [Crossref] [PubMed]
- Boonstra JJ, Koppert LB, Wijnhoven B, et al. Chemotherapy followed by surgery in patients with carcinoma of the distal esophagus and celiac lymph node involvement. J Surg Oncol 2009;100:407-13. [Crossref] [PubMed]
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [Crossref] [PubMed]
- Amini A, Xiao L, Allen PK, et al. Celiac node failure patterns after definitive chemoradiation for esophageal cancer in the modern era. Int J Radiat Oncol Biol Phys 2012;83:e231-9. [Crossref] [PubMed]


