Atrial fibrillation is related to lower incidence of deep venous thrombosis in patients with pulmonary embolism
Introduction
Pulmonary embolism (PE) is a potentially life-threatening condition, affecting a significant proportion of hospitalized patients (1). In Iceland, its incidence was reported to be 0.05%, whereas in Chinese populations estimated to be between 0.09% to 1.12% (1,2). PE is a known manifestation of venous thromboembolism (VTE). Approximately 70% to 80% of PE events are thought to be secondary to deep venous thromboses (DVTs) rather than a separate clinical entity (3,4). Risk factors for VTE are previous VTE, recent surgery or trauma, advanced age, prolonged immobilization, cancer, thrombophilia, acute myocardial infarction or stroke, congestive heart failure, etc. (1,2,5-8). However, these factors have variable predictive values of DVT and PE.
The association between left atrial appendage thrombosis and systemic embolism in atrial fibrillation (AF) patients is well recognized. Recent studies have pointed out the theoretical potential for a similar phenomenon for the right atrial appendage (RAA), which might also lead to thrombosis that could elevate the risk of PE (9,10). Thus, RAA thrombi have been detected in AF patients with PE reported as a complication of electrical and pharmacologic cardioversion of AF (11-13). However, there is little information on incidence of potential risk factors of PE in AF populations. Therefore, we conducted a retrospective study to investigate the most common risk factors and clinical characteristics of PE patients with and without AF.
Methods
Study population
This study complied fully by the declaration of Helsinki and was specifically approved by the Dalian Medical University Ethics Committee (Ethic Approval ID: YJ-KY-FB-2015-20). The study population included patients presenting to The First Affiliated Hospital of Dalian Medical University. They were identified by searching through hospital medical records with a coded diagnosis of PE with and without AF. From 2002 to 2015, total 10,540 and 1,709 patients were found with primary diagnosis of AF and PE respectively. Patients having 12-lead ECG or Holter evidence with previous history of AF and confirmed PE were categorized into the study group. Age and sex-matched PE patients without AF were enrolled into the control group. A case to control ratio of 1:2 was used.
PE: diagnosis and validation
Cases of PE were collected from medical records of patients and confirmed based established criteria. These included clinical signs and symptoms of dyspnea, chest pain, cyanosis, and pre-syncope confirmed by radiological evidence using computer tomography pulmonary angiography (CTPA), spiral computed tomography or perfusion-ventilation. The presence or absence of risk factors for PE was examined: DVT (diagnosed by compression ultrasonography or venography), recent surgery or trauma (previous 8 weeks) and active malignancy. In this study, the majority of patients received CTPA or spiral computed tomography. For patients with severe disease in whom we were unable to perform radiological testing, they satisfied of the following: the clinical signs and symptoms suggestive of PE, high D-dimer >500, hypoxemia with PaO2 ≤80 mmHg, with or without electrocardiographic signs of S1Q3T3, and the presence of signs of DVT.
Five independently trained personnel were assigned to review the medical data. To avoid bias, they were blinded to the study objectives. All information on study endpoints were validated by medical records and adjudicated by an independent review panel.
Study variables
Variables that were obtained at the baseline from discharge medical records were demographic and clinical details. Clinical variables included the most common risk factors of PE, including the new diagnosis of DVT, previous history of DVT, history of recent surgery or trauma within the preceding 8 weeks of presentation to hospital, active malignancy, pulmonary diseases and smoking. The CHA2DS2-VASc and CHADS2 scores were also determined. The CHADS2 score include the following components: congestive heart failure (1 point), hypertension (1 point), age ≥75 years (1 point), diabetes mellitus (1 point), and previous stroke/transient ischemic accident (2 points), while CHA2DS2-VASc score include: congestive heart failure (1 point), hypertension (1 point), age ≥75 years (2 points), diabetes mellitus (1 point), previous stroke/transient ischemic accident (2 points), vascular disease (1 point), age 65–75 years (1 point) and female gender (1 point) were calculated at the study entry for each patient (14,15). Both the CHADS2 and CHA2DS2-VASc scores include categories of 0= low risk, 1= intermediate risk, and ≥2= high risk (14,15). In order to evaluate better predictability for predicting the risk of PE in study group, CHADS2 and CHA2DS2-VASc scores were classified into two categories: low-intermediate risk (<2 points) and high-risk (≥2 points).
Drug history was also obtained from the pharmacy and discharge records. This included antithrombotic therapy with oral anticoagulants.
Statistical analysis
Data were expressed as frequencies and percentages. Continuous variables were summarized with mean +/− standard deviation and statistical comparison was made by Student’s t-test. The Chi square test was used to compare significant differences between two categories of CHADS2 and CHA2DS2-VASc scores. Statistical significance was considered at P<0.05. All statistical analyses were performed by SPSS version 23 (SPSS IBM).
Results
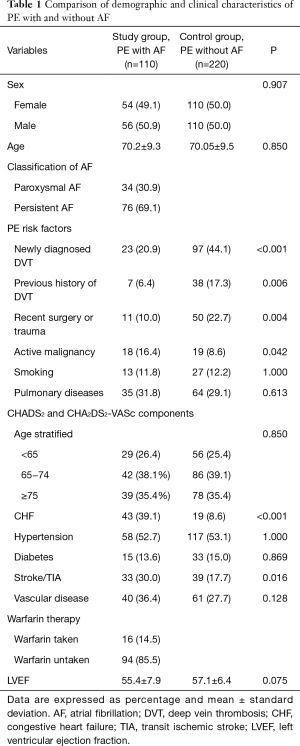
In a total of 10,540 patients of the hospital, 110 patients (1.1%) satisfied the inclusion criteria of diagnosed PE and AF for the study group (mean age 70.2±9.3 years). For the control group, 220 patients with diagnosis of PE without AF were matched (mean age 70.05±9.5 years). Table 1 lists the demographic and clinical characteristics of this study population.
Full table
Risk factors of PE
Among the most common risk factors of PE, the study group had significantly lower incidence of newly diagnosed DVT (20.9% vs. 44.1%, P<0.001), previous history of DVT (6.4% vs. 17.3%, P=0.006) and recent surgery or trauma (10.0% vs. 22.7%, P=0.004) as compared to control group (Table 1).
CHA2DS2-VASc vs. CHADS2 score
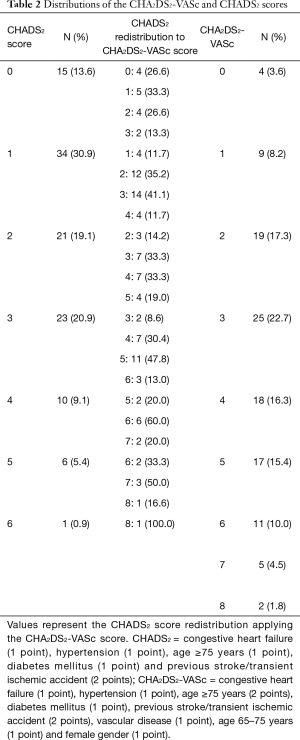
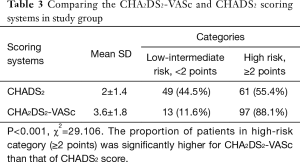
The CHADS2 and CHA2DS2-VASc scores are established score systems for stratification of stroke risk in AF patients (Table 1). The distribution of the CHADS2 and CHA2DS2-VASc scoring systems are display in Tables 2,3, respectively. Using the CHADS2 score, the most frequent score was 1 (30.9%) and 49 patients (44.5%) were placed in the low-intermediate risk (<2 points) category. By contrast, using the CHA2DS2-VASc score, the most frequent was 3 (22.7%) in study group. CHA2DS2-VASc arranged 13 (11.6%) patients (P<0.001) (Table 3). CHA2DS2-VASc score redistributed 36 (73.4%) patients in the CHADS2 low-intermediate risk category as high-risk (≥2 points) category, an indication for prescription of anticoagulant medications. These findings suggest that the CHA2DS2-VASc score has better predictability for predicting the low- and high-risk PE patients as compared to CHADS2. CHA2DS2-VASc score (≥2 points) might be associated with high-risk factor of PE in patients with AF.
Full table
Full table
Anticoagulation therapy
In terms of anticoagulation use in our study group, 94 patients (85.5%) did not receive any, whereas 16 patients (14.5%) were warfarin taken with a mean international normalized ratio (INR) of 1.87±0.85. Despite the fact that 97 patients (88.1%) had a CHA2DS2-VASc score of ≥2, only 16 (16.4%) patients received warfarin therapy. Out of the 16 patients who had warfarin therapy, ten had poor INR control within therapeutic range (≥2).
Discussion
The main findings of this study are that (I) PE patients with AF had significantly lower incidence of newly and previous history of DVT, and lower history of recent surgery or trauma when compared to patients with PE patients without AF; and (II) The CHA2DS2-VASc scoring system remained a better predictive tool for the high-risk of PE patients with AF, as compare to CHADS2.
Relationship between AF and PE
Previous studies have reported that peripheral thrombus could not be identified in up to 50% of PE patients (16,17). van Langevelde et al. found peripheral thrombus in only 44% of PE patients by advanced magnetic resonance imaging technique. The pulmonary emboli was suggested to be arose from the site other than the deep vein systems, such as thrombi of a right cardiac origin or de novo formation in the lung arteries (17).
In the present study, the incidence of DVT was significantly lower in the study group compared to the control group, and when compared with previous studies (16,17). The absence of thrombus secondary to DVTs for the PE event in the study group is consistent with the notion of additional causal factors, as reported previously. Thus, Keller et al. demonstrated that AF was the strongest discriminative condition and might play a role in the pathophysiology of PE without DVT (18). Sorensen et al. reported the AF patients in the preceding 3 months were associated with markedly increase risk of PE without coexisting peripheral DVT (19), which is consistent to our results.
Other studies have also reported a strong relationship between AF and PE (9-11,20-22). In the Tromsø study, patients with AF had an eight-fold risk for subsequent VTE particularly PE during first 6 months after AF diagnosis (HR =8.44, 95% Cl, 5.61–12.69) (9). Similarly, Wang et al. found the incidence of PE was significantly higher in AF group than non-AF group (21). The thrombogenic tendency had been explained and observed in both atria of AF patients as a cause of stroke and PE in several studies (11,21,23-25). Right atrium spontaneous echo contrast has been noted in AF patients without DVT, which could directly lead to pulmonary artery occlusion and consequent development of PE (24). Autopsy and echocardiographic studies also illustrated clot formation in the right atrium, predominantly at the RAA, in patients with AF (11,25,26). Their findings pointed out that right intra-cardiac thrombosis might be as common as left intra-cardiac thrombosis (26), despite the different anatomical characteristics of right and left atrium (11). Furthermore, some studies showed that the most of the right cardiac thrombi occurred in the RAA in patients with AF (10,11,26). Thus, AF patients might have an origin for clots in the right heart particularly appendage and linked to a potential causal and preceding risk factor of PE in absence of DVT.
CHA2DS2-VASc vs. CHADS2 score for prediction of PE
CHA2DS2-VASc and CHADS2 scores are well-recognized scores for stratification of stroke risk in AF patients. Of these, where 90% of thrombus is originated from left atrial appendage and CHA2DS2-VASc score has demonstrated better predictability (27). Most components of these stroke risk scoring systems are also risk factors for PE (8,26,28-32). However, for prediction of RAA thrombosis and incidence of PE, very few studies have been done to determine better predictability between CHA2DS2-VASc and CHADS2 scores in AF patients. Saliba et al. had found that high CHA2DS2-VASc score is directly associated with increased risk of PE among AF patients (32). Consistent with these findings, our study revealed that the CHA2DS2-VASc low-intermediate risk category had lower 13 (11.6%) vs. 49 (44.5%) and high-risk category had higher 97 (88.1%) vs. 61 (55.4%) proportion of patients compared to CHADS2 score. The CHA2DS2-VASc score is recommended for risk stratification of stroke in the 2014 guidelines of ESC and CCS as compared to classical CHADS2 score (33,34). The results of our study support the notion that CHA2DS2-VASc is a useful and suitable scoring system than CHADS2 score regarding prediction and stratification for the PE in patient with AF.
Anticoagulation
In our center, warfarin is common anticoagulant agent prescribed. Warfarin was only observed in 16 patients (14.5%). In 97 patients with CHA2DS2-VASc score of ≥2 (88.1%), only 16 (16.4%) received warfarin therapy. Of these, ten did not reach the target INR, which might underlie the high incidence of PE in the study group. Similar reports have been published that the use of anticoagulant was very low in Chinese hospitalized AF patients and in Chinese community based studies, even among high risk (CHA2DS2-VASc score ≥2) individuals (35-37). Public awareness and health education for thromboembolic event prevention with anticoagulant according to risk scores and current guidelines in AF patients are needed in China.
However, warfarin has been the mainstay anticoagulant agent for ischemic stroke and thromboembolic event prevention and could reduce the risk of stroke by 64% in AF patients (38). At the meantime, the application of anticoagulant was suggested to be highly beneficial and could reduce about 33% the risk of PE and 39% all-cause mortality rates in AF patients (32). Our results further supported that anticoagulation in high risk AF patients with CHA2DS2-VASc ≥2 are important not only for stroke prevention, but also for PE. Moreover, in many cases closure of left atrial appendage has been concerned to overcome the warfarin contraindications in AF patients (27,39). Whether RAA closure could reduce the incidence of PE and bleeding complications in AF patients remains further elucidation.
Limitations
The design of this work is a retrospective study carried out in a single hospital with a relatively small sample size. We have compared several factors to elucidate direct conclusion but due to nature of our study, it is not possible to draw direct conclusions on the causal association of AF and PE. In addition, our study did not have the power to explain that PE itself causes AF, where acute onset AF patients were excluded from study group. Further studies are warranted to conduct more details investigations.
Conclusions
The incidence of DVT in patients with PE and AF is lower than those without AF. These findings are consistent with the notion that the thrombi burden may not arise solely from the deep vein systems, but possibly from the right heart or are formed de novo within the large arteries. The CHA2DS2-VASc scoring system might be more sensitive for risk prediction and stratification of the PE in AF patients.
Acknowledgements
We hereby acknowledged all colleague clinicians who contributed in diverse ways during the entire study period.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study complied fully by the declaration of Helsinki and was specifically approved by the Dalian Medical University Ethics Committee (Ethic Approval ID: YJ-KY-FB-2015-20).
References
- Jonsson KO, Agnarsson UT, Danielsen R, et al. Pulmonary embolism at Landspitali, the National University Hospital of Iceland 2005-2007 - incidence, clinical manifestations, risk factors and outcome. Laeknabladid 2013;99:11-5. [PubMed]
- Liu XT, Lin GZ, Zhao XZ. Risk factors of pulmonary embolism among 303 patients in the First Clinical Hospital of Jilin University. Zhonghua Liu Xing Bing Xue Za Zhi 2011;32:1275-7. [PubMed]
- Girard P, Musset D, Parent F, et al. High prevalence of detectable deep venous thrombosis in patients with acute pulmonary embolism. Chest 1999;116:903-8. [Crossref] [PubMed]
- Elias A, Colombier D, Victor G, et al. Diagnostic performance of complete lower limb venous ultrasound in patients with clinically suspected acute pulmonary embolism. Thromb Haemost 2004;91:187-95. [PubMed]
- Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis 2016;41:3-14. [Crossref] [PubMed]
- Zakai NA, Callas PW, Repp AB, et al. Venous thrombosis risk assessment in medical inpatients: the medical inpatients and thrombosis (MITH) study. J Thromb Haemost 2013;11:634-41. [Crossref] [PubMed]
- Spyropoulos AC, Anderson FA Jr, FitzGerald G, et al. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest 2011;140:706-14. [Crossref] [PubMed]
- Nielsen JD. The incidence of pulmonary embolism during deep vein thrombosis. Phlebology 2013;28 Suppl 1:29-33. [Crossref] [PubMed]
- Enga KF, Rye-Holmboe I, et al. Atrial fibrillation and future risk of venous thromboembolism:the Tromso study. J Thromb Haemost 2015;13:10-6. [Crossref] [PubMed]
- Flegel KM. When atrial fibrillation occurs with pulmonary embolism, is it the chicken or the egg? CMAJ 1999;160:1181-2. [PubMed]
- de Divitiis M, Omran H, Rabahieh R, et al. Right atrial appendage thrombosis in atrial fibrillation: its frequency and its clinical predictors. Am J Cardiol 1999;84:1023-8. [Crossref] [PubMed]
- Carr B, Hawley K, Channer KS. Cardioversion of atrial fibrillation of recent onset with flecainide. Postgrad Med J 1991;67:659-62. [Crossref] [PubMed]
- Oram S, Davies JP. Further Experience of Electrical Conversion of Atrial Fibrillation to Sinus Rhythm: Analysis of 100 Patients. Lancet 1964;1:1294-8. [Crossref] [PubMed]
- Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263-72. [Crossref] [PubMed]
- Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864-70. [Crossref] [PubMed]
- Girard P, Sanchez O, Leroyer C, et al. Deep venous thrombosis in patients with acute pulmonary embolism: prevalence, risk factors, and clinical significance. Chest 2005;128:1593-600. [Crossref] [PubMed]
- van Langevelde K, Sramek A, Vincken PW, et al. Finding the origin of pulmonary emboli with a total-body magnetic resonance direct thrombus imaging technique. Haematologica 2013;98:309-15. [Crossref] [PubMed]
- Keller K, Prochaska JH, Coldewey M, et al. History of deep vein thrombosis is a discriminator for concomitant atrial fibrillation in pulmonary embolism. Thromb Res 2015;136:899-906. [Crossref] [PubMed]
- Sørensen HT, Horvath-Puho E, Lash TL, et al. Heart disease may be a risk factor for pulmonary embolism without peripheral deep venous thrombosis. Circulation 2011;124:1435-41. [Crossref] [PubMed]
- Bikdeli B, Abou Ziki MD, Lip GY. Pulmonary Embolism and Atrial Fibrillation: Two Sides of the Same Coin? A Systematic Review. Semin Thromb Hemost 2017;43:849-63. [Crossref] [PubMed]
- Wang CC, Lin CL, Wang GJ, et al. Atrial fibrillation associated with increased risk of venous thromboembolism. A population-based cohort study. Thromb Haemost 2015;113:185-92. [Crossref] [PubMed]
- Kukla P, McIntyre WF, Koracevic G, et al. Relation of atrial fibrillation and right-sided cardiac thrombus to outcomes in patients with acute pulmonary embolism. Am J Cardiol 2015;115:825-30. [Crossref] [PubMed]
- Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet 2009;373:155-66. [Crossref] [PubMed]
- Yasuoka Y, Naito J, Hirooka K, et al. Right atrial spontaneous echo contrast indicates a high incidence of perfusion defects in pulmonary scintigraphy in patients with atrial fibrillation. Heart Vessels 2009;24:32-6. [Crossref] [PubMed]
- Ogren M, Bergqvist D, Eriksson H, et al. Prevalence and risk of pulmonary embolism in patients with intracardiac thrombosis: a population-based study of 23 796 consecutive autopsies. Eur Heart J 2005;26:1108-14. [Crossref] [PubMed]
- Aberg H. Atrial fibrillation. I. A study of atrial thrombosis and systemic embolism in a necropsy material. Acta Med Scand 1969;185:373-9. [Crossref] [PubMed]
- Yu CM, Khattab AA, Bertog SC, et al. Mechanical antithrombotic intervention by LAA occlusion in atrial fibrillation. Nat Rev Cardiol 2013;10:707-22. [Crossref] [PubMed]
- Goldhaber SZ, Grodstein F, Stampfer MJ, et al. A prospective study of risk factors for pulmonary embolism in women. JAMA 1997;277:642-5. [Crossref] [PubMed]
- Dean SM, Abraham W. Venous thromboembolic disease in congestive heart failure. Congest Heart Fail 2010;16:164-9. [Crossref] [PubMed]
- Petrauskiene V, Falk M, Waernbaum I, et al. The risk of venous thromboembolism is markedly elevated in patients with diabetes. Diabetologia 2005;48:1017-21. [Crossref] [PubMed]
- Prandoni P, Bilora F, Marchiori A, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med 2003;348:1435-41. [Crossref] [PubMed]
- Saliba W, Rennert G. CHA2DS2-VASc score is directly associated with the risk of pulmonary embolism in patients with atrial fibrillation. Am J Med 2014;127:45-52. [Crossref] [PubMed]
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1-76. [Crossref] [PubMed]
- Macle L, Cairns JA, Andrade JG, et al. The 2014 Atrial Fibrillation Guidelines Companion: A Practical Approach to the Use of the Canadian Cardiovascular Society Guidelines. Can J Cardiol 2015;31:1207-18. [Crossref] [PubMed]
- Xiong Q, Shantsila A, Li J, et al. Suboptimal oral anticoagulant treatment among Chinese non-valvular atrial fibrillation patients: the Nanchang Atrial Fibrillation Project. Arch Med Sci 2016;12:216-8. [Crossref] [PubMed]
- Sun Y, Hu D. Chinese Investigators of GARFIELD, et al. Chinese subgroup analysis of the global anticoagulant registry in the FIELD (GARFIELD) registry in the patients with non-valvular atrial fibrillation. Zhonghua Xin Xue Guan Bing Za Zhi 2014;42:846-50. [PubMed]
- Zhou Z, Hu D. An epidemiological study on the prevalence of atrial fibrillation in the Chinese population of mainland China. J Epidemiol 2008;18:209-16. [Crossref] [PubMed]
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857-67. [Crossref] [PubMed]
- Holmes DR Jr, Doshi SK, Kar S, et al. Left Atrial Appendage Closure as an Alternative to Warfarin for Stroke Prevention in Atrial Fibrillation: A Patient-Level Meta-Analysis. J Am Coll Cardiol 2015;65:2614-23. [Crossref] [PubMed]


