Staging lymph node metastases from lung cancer in the mediastinum
Background
Lung cancer has remained one of the most devastating and deadliest cancers worldwide accounting for 18% of all cancer deaths (1). Imparting some of this lethality is lung cancer’s aggressive heterogeneous nature, often presenting in advanced stage where the five-year survival is less than 5% (2). This heterogeneity has also conveyed difficulties in properly staging lung cancer.
Now in its 7th edition, lung cancer staging has gone through several revisions collectively overseen by The International Staging Committee of the International Association for the Study of Lung Cancer (IASLC) and based on the “TNM” classification system. The newest edition has called for revisions of the “T” and “M” components, and after extensive review, found that tumor size had prognostic relevance and that a better differentiation of tumors produced patients with different prognoses (3). However, these evidence-based sub-classifications made no changes to the “N” component, which has remained relatively unchanged through several lung cancer staging revisions.
N2 nodal disease
Current classification of the “N” component sub-divides it into four divisions, no lymph node metastasis (N0), local peribronchial and/or ipsilateral hilar lymph node metastasis (N1), ipsilateral mediastinal and/or subcarinal lymph node metastasis (N2), and contralateral mediastinal and/or supraclavicular lymph node metastasis (N3). However, the N2 classification can be considered the most expansive as it corresponds with lymph node stations of the superior mediastinum (2R, 2L, 3A, 3P, 4R, and 4L) extending to the lower mediastinum [7, 8, and 9] and including those lymph nodes of the aortopulmonary window and para-aorta (5 and 6, respectively). Due to the broad region and number of lymph nodes that N2 disease comprises, it can lead to a heterogeneous mix of lung cancers that can have different survival rates (4,5). Although several changes have been proposed for re-classifying the “N” component, the diversity of “N” disease based on global geography and tumor biology, has made it difficult to obtain a consensus validation (3).
Amongst the proposed changes for N2 disease, IASLC has suggested the concept of nodal zones. This classification system arranges 14 lymph node stations into seven lymph node zones (3). Studies supporting this have shown that under this proposed system, patients with single N2 zone positivity have a significantly higher survival rate than patients with multiple N2 zones and have a prognosis similar to patients with multiple positive N1 lymph nodes (3). Other studies have proposed other “N” classification methods based on the number of positive metastatic lymph nodes (6,7), the ratio of metastatic lymph node number to the number of total lymph nodes resected (7-9), and to the combination of both number as well as rate of metastatic lymph nodes (10). Regardless of the kind of reclassification of N2 disease undertaken, any future revision will carry major clinical implications as mediastinal lymph node metastasis is one of the most important factors in determining lung cancer treatment. This is especially true for N2 disease, where metastatic status at time of lung cancer diagnosis can be seen as a “watershed” area between which modality or combination of modalities will be undertaken for treatment.
Moving towards molecular staging
The TNM staging system was established in 1958, and in lung cancer, it is based almost exclusively on determining the anatomic extent of the cancer based on disease burden and spread. Although the American Joint Commission on Cancer (AJCC) was largely responsible for its widespread adoption, the TNM system has now become the gold standard for international reporting of lung cancer staging as shown by its most recent refinement in the 2010 IASLC classification/staging reports (11). Since patient survival has long been associated with the anatomical extent of disease from the primary tumor, the TNM staging system has always proved strongly to correlate with lung cancer long-term survival rates (12). Moreover, not only has the clinical outcome of lung cancer been predicted based on this TNM staging, but also the treatment plans of individual patients have been prescribed by physicians based on anatomical extent of disease. It is generally accepted, for example, that local treatment modalities such as surgery and radiation therapy are inappropriate to administer for curative intent once the disease has spread beyond the surgical margins of resection or the confines of the radiation field, respectively. But, since the TNM staging system is anatomically based with visual inspection of tissue being critical, these management decisions about options of patient therapy and insights into prognostics have been until recently reliant on the skill of the pathologist and the optical power of a microscope.
In lung cancer staging through the years, histological type, differentiation, and clinical characteristics of patients such as age or race have not been fully incorporated into the TNM staging system. Recently, however, a worldwide effort, led by William Travis of Memorial Sloan Kettering, has re-examined the previous motley classification of adenocarcinoma histology to reveal distinct histological subtypes that do confer prognostic value (13). As others have suspected, this perhaps speaks to a strong correlation between histology and molecular determinants of lung cancer as exemplified in molecular features such as gene-expression profiles (14,15). In fact, the dawn of polymerase chain reaction (PCR) technology and the burgeoning field of molecular diagnostics are proving to be powerful technologies for determining the extent of cancer spread in pathological specimens. It is anticipated that they may fundamentally change the TNM staging system if accumulated evidence persuades their incorporation into the TNM staging system by the AJCC and/or the IASLC.
At present, most molecular prognostic markers in lung cancer have principally used only the T component of the staging system to estimate survival, such as recent published examples (Table 1). This concept is based on the hypothesis that the genes or proteins being identified in the primary tumor alone molecularly confer a certain clinical outcome due to their presence and function inherently, and that this is necessary and sufficient to determine tumor behavior. The problem with this approach is that it ignores the time tested benefits of all components of the TNM staging system. Instead of the intense focus on defining molecular signatures solely based on the tumor, strategies should also define molecular characterization of N2 lymph nodes and metastatic disease (serum). Eventually, this may enable more value to be added to the current anatomical TNM system.
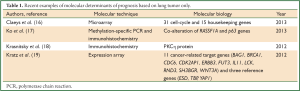
Full table
Some of the reasons for this shift away from examining molecular determinants of lymph node metastases are that early attempts to correlate molecular markers in mediastinal lymph nodes with clinical survival of lung cancer patients were largely unsuccessful (20,21). One of the largest efforts to date to incorporate a molecular evaluation of the N2 lymph nodes for occult, micrometastic tumor cells was performed in 2002 by the Cancer and Leukemia Group B Cooperative Cancer Group in the U.S.A. which failed to show any clinic benefit of molecular upstaging to patients (22).
Brock et al. recently advanced a step in the direction of molecular staging by proposing a set of four genes epigenetically modified that could be used to detect tumor DNA in N1 and N2 lymph nodes without evidence of visually discernible cancer cells, and which could be correlated with disease-free survival (23). Detecting tumor DNA rather than intact cells has an innate advantage because intact cells are needed to be visible in the mediastinal lymph nodes to ascertain the presence of cancer whereas tumor DNA may be present without microscopically observing tumor cells. Intact cancer cells are vulnerable to phagocytosis, especially if immune-inhibitory transmembrane receptors such as CD47 are not overexpressed, they can be fractured or fragmented by stress or trauma, and they can undergo apoptosis or necrosis for failure to implant into the nodal tissue. From any dead or dying cell, tumor DNA would be a residual product in the microenvironment fully available to be identified by PCR and detected for diagnostic purposes. Although molecular staging of the TNM system has not yet reached clinical relevance, the concept behind this approach is still both powerful and appealing. Future studies and more potent molecular marker technology may be needed to derive the full benefits of molecular staging of primary tumor and N2 lymph nodes.
Imaging modalities for evaluating N2 disease
Recent advancements in imaging modalities, such as computed tomography (CT) and positron emission tomography (PET), have drastically improved the detection and evaluation of lung cancer (24,25). CT imaging is now the most widely available and most commonly used imaging technique to assess intra- and extra-thoracic metastases (26). However, CT imaging has been shown to have limited abilities when evaluating the mediastinum for metastases when used as the sole modality. Investigations have shown that the sensitivity and specificity of CT imaging in identifying mediastinal metastases are 55% and 81%, respectively (26).
PET imaging, especially in combination with CT imaging, plays a prominent role in the evaluation of patients with lung cancer and is recommended preoperatively for most patients suspected of having lung cancer (26). Multiple investigations have assessed the validity of PET in identifying and evaluating mediastinal metastases (26-28). In comparison to CT imaging, PET has shown to have significantly better sensitivities and specificities, 77% and 86% respectively, when evaluating for mediastinal metastasis.
However, PET imaging, even when combined with CT, is not without its disadvantages. In areas of endemic granulomatous disease, such as sarcoidosis, HIV infection, and fungal disease, such as histoplasmosis, PET has been shown to increase the rates of false positive malignancy in mediastinal lymph nodes due to the increased metabolic activity these diseases engender in N2 lymph nodes (28-31). False mediastinal lymph node positivity on a PET scan will incorrectly upstage disease, which can erroneously direct patients from curative surgery (26,32). Hence, clinicians must be aware that PET imaging is not a definitive test and tissue confirmation is often needed to confirm PET scan findings. Despite its major positive impact on the stage classification of patients at a higher risk of having distant metastases outside the thorax, when used alone without tissue confirmation, PET imaging has the potential to be harmful if used in less structured settings.
Invasive techniques for evaluating N2 disease
Mediastinoscopy
Confirming mediastinal involvement is crucial in the treatment and prognosis of lung cancer. Non-invasive methods for establishing mediastinal involvement, such as PET/CT, are excellent in detecting disease, but do not provide definitive disease confirmation. A plethora of invasive techniques are now available to obtain tissue as the next step to confirm mediastinal metastases.
Mediastinoscopy has long been viewed as the “gold standard” for diagnostic evaluation of the lymph nodes of the mediastinum. Performed in an operative suite under general anesthesia, the procedure involves an incision just above the suprasternal notch, with insertion of a mediastinal scope alongside the trachea, allowing for biopsies of the mediastinal lymph nodes. Using this approach, lymph nodes stations 1, 2R, 2L, 3, 4R, 4L, and anterior station 7 lymph nodes can be sampled. The use of a video mediastinoscope may allow for greater sampling, such as access to the posterior lymph nodes of station 7, and possible performance of a lymph node dissection (33).
Mediastinoscopy may also be modified to sample the aortopulmonary lymph nodes of stations 5 and 6, such as in an extended cervical mediastinoscopy. In this procedure, using the same cervical incision as a traditional Mediastinoscopy, the mediastinal scope is directed laterally toward the aortic arch (34). However, due to the grave complications of this technique, extended cervical mediastinoscopies are delegated to the few institutions that routinely preform them (35-37).
Endobronchial and endoscopic ultrasound guided biopsies
Despite its low rates of morbidity and mortality, 2% and 0.08% respectively (38), the role of mediastinoscopy is changing in favor of less invasive techniques, such as endobronchial ultrasound (EBUS) guided biopsy and esophageal endoscopic ultrasound guided (EUS) biopsy. EBUS has been increasingly used in the staging of lung cancer due to its excellent diagnostic performance (26,39-41). EBUS biopsy was found to be significantly more sensitive for detecting malignant lymph nodes than transbronchial needle aspiration, 69% vs. 36% respectively (39). Overall, in patients who had clinical indications for an invasive investigation of the mediastinum, EBUS was shown to have a sensitivity of 89% with a negative predictive value of 91% (26).
Once considered a complimentary procedure of the mediastinoscopy, EUS biopsy has emerged as a viable alternative (39,42-44). Performed with minimal risks of complications, EUS has been shown to be particularly helpful in evaluating the lymph nodes of station 5 and the lymph nodes of the inferior mediastinum. EUS biopsy is also capable of obtaining tissue from outside the thorax to evaluate distant metastases, such as in the liver, celiac lymph nodes, and areas of the sub-diaphragm (44,45). When used for the detection of metastases to the mediastinum, EUS has been shown to have sensitivities and specificities as high as 89% and 100%, respectively (26).
Currently, and with the support of multiple investigations, EBUS and EUS are now being routinely combined to allow for near complete evaluation of the mediastinum (26,39,46,47). In a meta-analysis of seven studies comprising 811 patients with a lung cancer prevalence of 33%, EBUS plus EUS was able to produce 91% sensitivity and 100% specificity (26). However, despite their high appeal as alternatives to mediastinoscopy as a first line status in evaluating the mediastinum, they both require high levels of expertise to be performed effectively. Additionally, few clinicians are sufficiently trained to do both procedures well, so that two separate qualified clinicians are needed to carry out both procedures.
Intra-operative techniques: lymph node dissection vs. sampling
In the thoracic surgery literature, there has been a long running debate concerning the correct surgical technique of harvesting hilar and mediastinal lymph nodes from lung cancer patients during surgical resection. At the heart of the debate, is whether a small sampling of relevant lymph nodes is adequate or whether a complete dissection of all visible lymph nodes is needed.
Ludwig et al. added fuel to the fire with a population-based Surveillance, Epidemiology and End Results (SEER) study from 1990 to 2000 based on 16,800 patients with stage 1 NSCLC treated with surgical resection with curative intent which suggested that patient survival was associated with the number of lymph nodes evaluated pathologically for disease (48). Specifically, those patients with 13-16 lymph nodes examined by a pathologist had the best survival as compared to those with only 1-4 lymph nodes harvested (HR 0.78; 95% CI, 0.68-0.90). Surgical procurement of more than 16 lymph nodes did not seem to confer additional benefit. The authors concluded that this was most likely due to “a reduction-of-staging error”, in other words, that as more lymph nodes are sampled, there is a decreased tendency of a pathologist to miss any positive lymph nodes present.
Others have validated these findings for stage 1a lung cancers surgically resected in California, and in those states recorded in the SEER national registry (49,50). Additionally, complete mediastinal lymphadenectomy has been shown to be the most accurate mode of detecting multilevel N2 disease and skipped metastases (Pathologically positive N2 lymph nodes are present, but there is no evidence of histologically involvement of N1 lymph nodes) (51-55). Moreover, there has been concern that only 57% of patients undergoing major pulmonary resection for lung cancer have mediastinal lymph nodes harvested by their surgeon (56).
Darling et al. have largely settled this debate, at least for the time being, in a large randomized cooperative group trial that showed no difference between the survival of patients whose lymph nodes were procured by either of the two techniques (57). Interestingly, in both the right and left sides of the chest, a median of 18 lymph nodes were harvested per patient (12 N2 nodes and 6 N1 nodes). Based on this study, the cooperative group recommended that a surgeon procure, in addition to the tumor specimen and any N1 lymph nodes associated with it, at least 12 mediastinal lymph nodes during a mediastinal lymphadenectomy from stations 2R, 4R, 7, 8, 9, and 10R in the right chest, and stations 4L, 5, 6, 7, 8, 9, and 10L in the left chest. Finally, as more minimally invasive video assisted thoracic surgery (VATS), (especially VATS lobectomies) are being performed, the cooperative group study suggests a note of caution in that VATS lobectomies in their study were associated with fewer lymph nodes harvested per patient with a median of 15 versus 18/19 lymph nodes from an open thoracotomy (57).
Sentinel lymph node staging
Due to the morbidity of mediastinal lymph node dissection, over the last two decades, there has been an interest in developing a less invasive, more regional mode of determining pathological mediastinal lymph node status by examining a few sentinel lymph nodes. Importantly, this technique has been successful in other solid tumors (58). Sentinel node mapping is very much reliant on lymphatic flow drainage patterns, and the level at which lymph nodes are first impacted by drainage from the primary tumor bed. Recently, a systemic review of the literature on the efficacy of sentinel lymph node staging found that in relation to the proximity from the primary tumor the more distal N2 lymph nodes rather than the closer N1 nodes were the first sites of lymphatic drainage in a wide range of patient distributions ranging from 5% to 95% (59). This exemplifies the difficulty in the sentinel lymph node technique as the current technology of radiotracers and/or dyes shows a large variability in lymphatic drainage among patients as clinically observed with the phenomenon of “skipped metastases”.
Conclusions
Despite the current inadequacies in assessing N2 nodal disease in lung cancer, recent improvements on multiple fronts are allowing better prognostic and predictive information for treating patients. Studies aimed at reclassifying the anatomy, incorporating molecular determinants, improving the technology for imaging and of procuring the nodes, and finally advancing the pathologically assessment of N2 nodes will continue to push the envelope of science forward. Collectively, these multidisciplinary, cooperative efforts will enable patients to be treated more effectively, and hopefully lead to fewer deaths from this terrible disease.
Acknowledgements
Funding: Supported by Grant Number 3T32CA126607-04S1 and P50 CA058184 from the National Cancer Institute of the National Institutes of Health. Also, the American Association for Cancer Research/Stand Up to Cancer Dream Team Translational Cancer Research Grant, grant number SU2C-AACR-DT0109.
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. eds. SEER Cancer Statistics Review, 1975-2008, National Cancer Institute. Bethesda, MD. Available online: http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011.
- Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging for lung cancer. Ann Thorac Cardiovasc Surg 2009;15:4-9. [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Asamura H, Nakayama H, Kondo H, et al. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg 1999;117:1102-11. [PubMed]
- Wei S, Asamura H, Kawachi R, et al. Which is the better prognostic factor for resected non-small cell lung cancer: the number of metastatic lymph nodes or the currently used nodal stage classification? J Thorac Oncol 2011;6:310-8. [PubMed]
- Bria E, Milella M, Sperduti I, et al. A novel clinical prognostic score incorporating the number of resected lymph-nodes to predict recurrence and survival in non-small-cell lung cancer. Lung Cancer 2009;66:365-71. [PubMed]
- Saji H, Tsuboi M, Yoshida K, et al. Prognostic impact of number of resected and involved lymph nodes at complete resection on survival in non-small cell lung cancer. J Thorac Oncol 2011;6:1865-71. [PubMed]
- Wisnivesky JP, Arciniega J, Mhango G, et al. Lymph node ratio as a prognostic factor in elderly patients with pathological N1 non-small cell lung Cancer. Thorax 2011;66:287-93. [PubMed]
- Ito M, Yamashita Y, Tsutani Y, et al. Classifications of n2 non-small-cell lung cancer based on the number and rate of metastatic mediastinal lymph nodes. Clin Lung Cancer 2013;14:651-7. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Mountain CF. A new international staging system for lung cancer. Chest 1986;89:225S-233S. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol 2008;32:810-27. [PubMed]
- Sun Z, Yang P. Gene expression profiling on lung cancer outcome prediction: present clinical value and future premise. Cancer Epidemiol Biomarkers Prev 2006;15:2063-8. [PubMed]
- Claeys L, Iaccino F, Janssen CR, et al. Development and validation of a quantitative structure-activity relationship for chronic narcosis to fish. Environ Toxicol Chem 2013;32:2217-25. [PubMed]
- Ko E, Lee BB, Kim Y, et al. Association of RASSF1A and p63 with poor recurrence-free survival in node-negative stage I-II non-small cell lung cancer. Clin Cancer Res 2013;19:1204-12. [PubMed]
- Krasnitsky E, Baumfeld Y, Freedman J, et al. PKCη is a novel prognostic marker in non-small cell lung cancer. Anticancer Res 2012;32:1507-13. [PubMed]
- Kratz JR, He J, Van Den Eeden SK, et al. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet 2012;379:823-32. [PubMed]
- Ahrendt SA, Yang SC, Wuc L, et al. Molecular assessment of lymph nodes in patients with resected stage I non-small cell lung cancer: Preliminary results of a prospective study. J Thorac Cardiovasc Surg 2002;123:466-73, discussion 473-4. [PubMed]
- Harden SV, Tokumaru Y, Westra WH, et al. Gene promoter hypermethylation in tumors and lymph nodes of stage I lung cancer patients. Clin Cancer Res 2003;9:1370-5. [PubMed]
- D’cunha J, Corfits AL, Herndon Ii JE, et al. Molecular staging of lung cancer: real-time polymerase chain reaction estimation of lymph node micrometastatic tumor cell burden in stage I non-small cell lung cancer--preliminary results of Cancer and Leukemia Group B Trial 9761. J Thorac Cardiovasc Surg 2002;123:484-91. [PubMed]
- Brock MV, Hooker CM, Ota-Machida E, et al. DNA methylation markers and early recurrence in stage I lung cancer. N Engl J Med 2008;358:1118-28. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- Gould MK, Maclean CC, Kuschner WG, et al. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 2001;285:914-24. [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung Cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Sanli M, Isik AF, Zincirkeser S, et al. Reliability of positron emission tomography-computed tomography in identification of mediastinal lymph node status in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;138:1200-5. [PubMed]
- Birim O, Kappetein AP, Stijnen T, et al. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg 2005;79:375-82. [PubMed]
- Deppen S, Putnam JB Jr, Andrade G, et al. Accuracy of FDG-PET to diagnose lung Cancer in a region of endemic granulomatous disease. Ann Thorac Surg 2011;92:428-32; discussion433.
- Farrell MA, Mcadams HP, Herndon JE, et al. Non-small cell lung cancer: FDG PET for nodal staging in patients with stage I disease. Radiology 2000;215:886-90. [PubMed]
- Prabhakar HB, Rabinowitz CB, Gibbons FK, et al. Imaging features of sarcoidosis on MDCT, FDG PET, and PET/CT. AJR Am J Roentgenol 2008;190:S1-6. [PubMed]
- Modini C, Passariello R, Iascone C, et al. TNM staging in lung Cancer: role of computed tomography. J Thorac Cardiovasc Surg 1982;84:569-74. [PubMed]
- Leschber G, Sperling D, Klemm W, et al. Does video-mediastinoscopy improve the results of conventional mediastinoscopy? Eur J Cardiothorac Surg 2008;33:289-93. [PubMed]
- Ginsberg RJ. Extended cervical mediastinoscopy. Chest Surg Clin N Am 1996;6:21-30. [PubMed]
- Freixinet Gilart J, García PG, de Castro FR, et al. Extended cervical mediastinoscopy in the staging of bronchogenic carcinoma. Ann Thorac Surg 2000;70:1641-3. [PubMed]
- Metin M, Citak N, Sayar A, et al. The role of extended cervical mediastinoscopy in staging of non-small cell lung cancer of the left lung and a comparison with integrated positron emission tomography and computed tomography: does integrated positron emission tomography and computed tomogr. J Thorac Oncol 2011;6:1713-9. [PubMed]
- Call S, Rami-Porta R, Serra-Mitjans M, et al. Extended cervical mediastinoscopy in the staging of bronchogenic carcinoma of the left lung. Eur J Cardiothorac Surg 2008;34:1081-4. [PubMed]
- Kiser AC, Detterbeck FC. General aspects of surgical treatment. In: Detterbeck FC, Rivera MP, Socinski MA, et al. eds. Diagnosis and Treatment of Lung Cancer: an Evidence-Based Guide for the Practicing Clinician. Philadelphia, PA: WB Saunders, 2001:133-47.
- Wallace MB, Pascual JM, Raimondo M, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA 2008;299:540-6. [PubMed]
- Herth FJ, Eberhardt R, Krasnik M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest 2008;133:887-91. [PubMed]
- Lee BE, Kletsman E, Rutledge JR, et al. Utility of endobronchial ultrasound–guided mediastinal lymph node biopsy in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 2012;143:585-90. [PubMed]
- Larsen SS, Vilmann P, Krasnik M, et al. Endoscopic ultrasound guided biopsy versus mediastinoscopy for analysis of paratracheal and subcarinal lymph nodes in lung cancer staging. Lung Cancer 2005;48:85-92. [PubMed]
- Wallace MB, Ravenel J, Block MI, et al. Endoscopic ultrasound in lung cancer patients with a normal mediastinum on computed tomography. Ann Thorac Surg 2004;77:1763-8. [PubMed]
- Larsen SS, Vilmann P, Krasnik M, et al. Endoscopic ultrasound guided biopsy performed routinely in lung cancer staging spares futile thoracotomies: Preliminary results from a randomised clinical trial. Lung Cancer 2005;49:377-85. [PubMed]
- Annema JT, Versteegh MI, Veseliç M, et al. Endoscopic ultrasound added to mediastinoscopy for preoperative staging of patients with lung cancer. JAMA 2005;294:931-6. [PubMed]
- Herth FJ, Krasnik M, Kahn N, et al. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest 2010;138:790-4. [PubMed]
- Ohnishi R, Yasuda I, Kato T, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal nodal staging of lung cancer. Endoscopy 2011;43:1082-9. [PubMed]
- Ludwig MS, Goodman M, Miller DL, et al. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest 2005;128:1545-50. [PubMed]
- Ou SH, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J Thorac Oncol 2008;3:880-6. [PubMed]
- Varlotto JM, Recht A, Nikolov M, et al. Extent of lymphadenectomy and outcome for patients with stage I nonsmall cell lung cancer. Cancer 2009;115:851-8. [PubMed]
- Massard G, Ducrocq X, Kochetkova EA, et al. Sampling or node dissection for intraoperative staging of lung cancer: a multicentric cross-sectional study. Eur J Cardiothorac Surg 2006;30:164-7. [PubMed]
- Keller SM, Adak S, Wagner H, et al. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Ann Thorac Surg 2000;70:358-65. [PubMed]
- Wu Y, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6. [PubMed]
- Doddoli C, Aragon A, Barlesi F, et al. Does the extent of lymph node dissection influence outcome in patients with stage I non-small-cell lung cancer? Eur J Cardiothorac Surg 2005;27:680-5. [PubMed]
- Izbicki JR, Passlick B, Karg O, et al. Impact of radical systematic mediastinal lymphadenectomy on tumor staging in lung cancer. Ann Thorac Surg 1995;59:209-14. [PubMed]
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6; discussion 2056. [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [PubMed]
- Leong SP, Zuber M, Ferris RL, et al. Impact of nodal status and tumor burden in sentinel lymph nodes on the clinical outcomes of cancer patients. J Surg Oncol 2011;103:518-30. [PubMed]
- Taghizadeh Kermani A, Bagheri R, Tehranian S, et al. Accuracy of sentinel node biopsy in the staging of non-small cell lung carcinomas: systematic review and meta-analysis of the literature. Lung Cancer 2013;80:5-14. [PubMed]


