Comparison of exogenous and endogenous lipoid pneumonia: the relevance to bronchial anthracofibrosis
Lipoid pneumonia, caused by the accumulation of lipids in the alveoli, is an uncommon inflammatory lung disease (1). According to the source of the lipid exposure, there are two forms: exogenous and endogenous lipoid pneumonia (2,3). The exogenous form results from aspiration or inhalation of various types of lipid (4-7), whereas the endogenous form usually occurs in the setting of bronchial obstruction or other miscellaneous conditions (8-11). Most of the literature regarding lipoid pneumonia are sporadic case reports or series including only one of the two forms because of its rarity (5-11). There are no data directly comparing patients with exogenous and endogenous lipoid pneumonia. Thus, the clinical, radiologic, and bronchoscopic findings for exogenous and endogenous lipoid pneumonia were compared in this study.
All patients who were diagnosed with lipoid pneumonia between January 2008 and December 2016 at Kyungpook National University Hospital, a tertiary referral hospital in South Korea, were included. A diagnosis of lipoid pneumonia was confirmed if all of the following criteria were met (2): the presence of abnormal imaging features compatible with the findings of lipoid pneumonia on chest computed tomography (CT); the detection of lipid-laden macrophages in bronchoalveolar lavage (BAL) fluid or lung tissue on using a fat stain (oil-red O); and exclusion of other relevant diseases potentially explaining the radiologic abnormality. Patients were classified as having either the endogenous or exogenous form on the basis of a clinical history congruent with an exogenous origin of the lipid. Details on the clinical, radiologic, and bronchoscopic findings of the patient were collected.
The chest CT findings were evaluated as follows: ground glass opacity (GGO), consolidation, ill-defined nodules, septal thickening, pleural effusion, mediastinal lymphadenopathy, affected area, distribution, and extent (1,6). The extent of affected lesion was classified as follows: (I) within one segment; (II) within one lobe; and (III) beyond one lobe. Bronchoscopic findings were assessed in terms of the presence or absence of endobronchial lesion including anthracofibrosis. Bronchial anthracofibrosis (BAF) was defined as bronchial narrowing with multiple areas of dark anthracotic pigmentation on large airway mucosa (12,13).
During the study period, 18 patients were initially identified as having lipoid pneumonia by searching for the International Classification of Diseases 10th Revision Clinical Modification, code J691. Finally, a total of 13 patients received a confirmed diagnosis of lipoid pneumonia based on the criteria described above. The clinical, radiologic, and bronchoscopic data of patients are presented in Table 1. Eight (62%) patients were male, and the median age was 68 years (range, 51–83 years). In 12 patients, lipid-laden macrophages, with or without intra-alveolar lipid, were detected in both the BAL fluid and transbronchial lung biopsy tissue. One patient (case 2), who did not undergo lung biopsy, demonstrated macrophages positive for lipid staining in the BAL fluid. Eight patients (cases 1–8) with a history of oil ingestion and five patients (cases 9–13) without an identified history of oil exposure were diagnosed with exogenous and endogenous lipoid pneumonia, respectively. All patients with the exogenous form reported exposure to animal fats during history taking. None of the patients had a history of taking amiodarone, including the one case with atrial fibrillation. The median time between exposure and presentation was 12 months (range, 1 month–20 years). Nine (69%) patients had at least one comorbidity (cases 1–5, 8, 10, 11, and 13). Gastroesophageal reflux disease was noted in 3 (38%) patients with the exogenous form (cases 1, 3, and 8) and 1 (20%) patient with the endogenous form (case 13). Respiratory symptoms were present in five of eight patients with the exogenous form and all five patients with the endogenous form; cough and dyspnea were the most common respiratory complaints. All patients with the endogenous form presented with dyspnea, which was more frequent than those with the exogenous form (P=0.021). The presence of mycobacterial infection was excluded based on the results of microbiological tests including acid-fast bacilli smear, polymerase chain reaction, and culture using bronchial aspirate in all the patients.
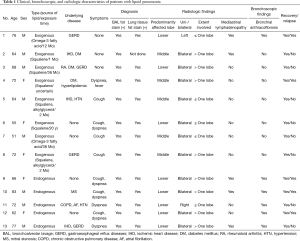
Full table
The most common CT findings were GGO (n=13) and septal thickening (n=13), followed by consolidation (exogenous, n=5; endogenous, n=2), ill-defined nodules (exogenous, n=4; endogenous, n=3), mediastinal lymphadenopathy (exogenous, n=2; endogenous, n=3), and pleural effusion (exogenous, n=1). These findings were comparable between both groups. Overall, 8 (62%) patients showed an extent of disease affecting one or more lobes. The lower lobe (62%) was the most frequently involved site, followed by the middle lobe (38%). There were no patients with an upper lobe predominance. All patients with the endogenous form showed a predilection for the involvement of the lower lobes, whereas the middle lobes (63%) were most frequently affected in patients with the exogenous form (P=0.075). Bilateral involvement (85%) was common in both forms.
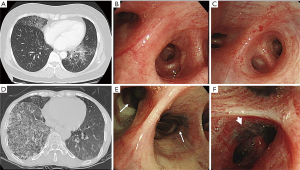
Figure 1A,B,C (case 6) and Figure 1D,E,F (case 12) are representative radiologic and bronchoscopic findings for the exogenous and endogenous forms, respectively. BAF, as shown in Figure 1E,F, was noted in all patients with endogenous lipoid pneumonia, whereas it was found in only one patient with the exogenous form (P=0.005). These findings may have caused the dyspnea at presentation in all patients with the endogenous form. All stenotic bronchi, due to anthracofibrosis, connected to the segment(s) or lobe(s) affected by lipoid pneumonia (Figure 1D,E,F) in patients with the endogenous form. Only one patient with the exogenous form (case 1) had some anthracotic pigmentation on the mucosa of multiple segmental bronchi with partial narrowing. However, the narrowed bronchus (superior segment of the left lower lobe) did not coincide with the site affected by lipoid pneumonia (anterior, lateral, and posterior basal segments of the left lower lobe). No other endobronchial lesions were identified bronchoscopically in both groups. Oil exposure was discontinued in patients with the exogenous form. All patients were treated with corticosteroids. In most, the starting prednisolone dose of 30 mg/day was tapered over several weeks. The median duration of treatment was 104 days (range, 48–152 days). All patients experienced resolution of symptoms and slow improvement of radiologic abnormalities over time. During the follow-up period (median, 426 days; range, 106–3,125 days), five patients (38%) experienced relapse of lipoid pneumonia. This total included two patients with the exogenous form (25%) who resumed taking omega-3 fatty acids (case 1) and squalene (case 4) and 3 patients (60%) with the endogenous form. In particular, two patients with the endogenous form experienced three or more recurrent episodes. The site of relapse coincided with that of the first episode in all patients with the endogenous form, whereas one patient with relapse of the exogenous form was affected at a site different from that of the first episode.
The present study highlights some differences between the two forms of lipoid pneumonia: dyspnea at presentation, predominant lower lobes involvement, BAF, and recurrent episodes were more frequent in patients with the endogenous form than in those with the exogenous form. Of them, the presence of BAF is the most striking difference between the two forms. The different clinical presentations and outcomes between the two forms may be because of BAF.
BAF, an obstructive pulmonary disease due to long-standing heavy exposure to biomass fuel smoke, is known to be associated with tuberculosis, pneumonia, chronic obstructive pulmonary disease, and lung cancer (12,13). The association between BAF and lipoid pneumonia is yet to be reported. Endogenous lipoid pneumonia usually develops in association with bronchial obstruction, such as lung cancers (8,14,15), although many other conditions, including chronic pulmonary infection, lipid-storage disorder, and sclerosing cholangitis, have been described in association with this disease (9-11,16). In the present study, patients with the endogenous form did not have any of the conditions mentioned above, except bronchial obstruction due to BAF. Thus, it seems plausible that bronchial obstruction due to anthracofibrosis may also be associated with endogenous lipoid pneumonia. In addition, frequent relapse episodes (60%) at the same site as the initial disease in patients with the endogenous form support the link between endogenous lipoid pneumonia and the pathogenic role of irreversible, persistent bronchial stenosis due to anthracofibrosis. Further study is needed to elucidate the relationship between BAF and endogenous lipoid pneumonia.
With regard to the frequently involved sites in patients with lipoid pneumonia who were included in this study, most cases with the exogenous form had bilateral (88%) involvement and affected the middle lobe (n=5) predominantly, followed by the lower lobes (n=3); however, the middle and lower lobes were simultaneously involved in the five patients with middle lobe predominance. This finding is in line with the results of previous studies that lipoid pneumonia caused by oil aspiration or inhalation has a predilection for involvement of the middle and lower lobes and multi-lobar involvement (3-6). On the other hand, the involvement site in patients with the endogenous form may differ based on the presence of local or systemic factors (3).
The clinical management practices in patients with lipoid pneumonia include avoiding ongoing exposure in case of the exogenous form and providing supportive care (2). The use of steroid therapy remains controversial although there are anecdotal reports on the beneficial effect of corticosteroids (2,17,18). In this study, a low dose of prednisolone (<0.5 mg/kg) was used in all the patients. As a result, all the patients showed improvement of symptoms and radiologic abnormalities without significant adverse events; however, the possibility of spontaneous improvement cannot be ruled out. Further study is required on the use of corticosteroids in patients with this disease.
The major limitations of our study were the small sample size and possible selection bias. The study was insufficient to allow complete comparison of the clinical and radiologic findings between the two forms of lipoid pneumonia. However, to our knowledge, there has been no report of a comparison of the two forms of lipoid pneumonia, especially in terms of bronchoscopic findings. In addition, the presence of BAF in all patients with endogenous lipoid pneumonia suggests that endogenous lipoid pneumonia should at least be considered in the differential diagnosis for patients with abnormal radiologic infiltration accompanied by BAF, which does not respond to conventional management.
In conclusion, there were some differences in the clinical manifestations and outcomes between exogenous and endogenous lipoid pneumonia. Notably, BAF was identified in all five patients with the endogenous form; however, it was only observed in one of the eight patients with the exogenous form. These results suggest that BAF may be associated with endogenous lipoid pneumonia and the different clinical presentations and outcomes in exogenous and endogenous lipoid pneumonia. Awareness of this association between endogenous lipoid pneumonia and BAF may help clinicians avoid missing or delaying diagnoses.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Betancourt SL, Martinez-Jimenez S, Rossi SE, et al. Lipoid pneumonia: spectrum of clinical and radiologic manifestations. AJR Am J Roentgenol 2010;194:103-9. [Crossref] [PubMed]
- Hadda V, Khilnani GC. Lipoid pneumonia: an overview. Expert Rev Respir Med 2010;4:799-807. [Crossref] [PubMed]
- Sundberg RH, Kirschner KE, Brown MJ. Evaluation of lipoid pneumonia. Dis Chest 1959;36:594-601. [Crossref] [PubMed]
- Marchiori E, Zanetti G, Mano CM, et al. Exogenous lipoid pneumonia. Clinical and radiological manifestations. Respir Med 2011;105:659-66. [Crossref] [PubMed]
- Gondouin A, Manzoni P, Ranfaing E, et al. Exogenous lipid pneumonia: a retrospective multicentre study of 44 cases in France. Eur Respir J 1996;9:1463-9. [Crossref] [PubMed]
- Laurent F, Philippe JC, Vergier B, et al. Exogenous lipoid pneumonia: HRCT, MR, and pathologic findings. Eur Radiol 1999;9:1190-6. [Crossref] [PubMed]
- Kuroyama M, Kagawa H, Kitada S, et al. Exogenous lipoid pneumonia caused by repeated sesame oil pulling: a report of two cases. BMC Pulm Med 2015;15:135. [Crossref] [PubMed]
- De Navasquez S, Haslewood GA. Endogenous lipoid pneumonia with special reference to carcinoma of the lung. Thorax 1954;9:35-7. [Crossref] [PubMed]
- Berghaus TM, Haeckel T, Wagner T, et al. Endogenous lipoid pneumonia associated with primary sclerosing cholangitis. Lancet 2007;369:1140. [Crossref] [PubMed]
- Itoh Y, Segawa H, Kito K, et al. Lipoid pneumonia with chronic myelomonocytic leukemia. Pathol Res Pract 2009;205:143-7. [Crossref] [PubMed]
- Nicholson AG, Wells AU, Hooper J, et al. Successful treatment of endogenous lipoid pneumonia due to Niemann-Pick Type B disease with whole-lung lavage. Am J Respir Crit Care Med 2002;165:128-31. [Crossref] [PubMed]
- Gupta A, Shah A. Bronchial anthracofibrosis: an emerging pulmonary disease due to biomass fuel exposure. Int J Tuberc Lung Dis 2011;15:602-12. [Crossref] [PubMed]
- Assad NA, Kapoor V, Sood A. Biomass smoke exposure and chronic lung disease. Curr Opin Pulm Med 2016;22:150-7. [Crossref] [PubMed]
- Sulkowska M, Sulkowski S, Chyczewski L, et al. Surfactant system in lung cancer. Endogenous lipid pneumonia. Neoplasma 1997;44:167-71. [PubMed]
- Cohen AB, Cline MJ. In vitro studies of the foamy macrophage of postobstructive endogenous lipoid pneumonia in man. Am Rev Respir Dis 1972;106:69-78. [Crossref] [PubMed]
- Byerley JS, Hernandez ML, Leigh MW, et al. Clinical approach to endogenous lipoid pneumonia. Clin Respir J 2016;10:259-63. [Crossref] [PubMed]
- Chin NK, Hui KP, Sinniah R, et al. Idiopathic lipoid pneumonia in an adult treated with prednisolone. Chest 1994;105:956-7. [Crossref] [PubMed]
- Ayvazian LF, Steward DS, Merkel CG, et al. Diffuse lipoid pneumonitis successfully treated with prednisone. Am J Med 1967;43:930-4. [Crossref] [PubMed]


