Clinical implication of the innovations of the 8th edition of the TNM classification for esophageal and esophago-gastric cancer
Introduction
For the past two decades, epidemiology of esophageal cancer and esophagogastric junction (EGJ) has deeply changed. Adenocarcinoma has increased dramatically in North America and in Europe concerning more 60 percent of all esophageal cancers in the United States. In contrast, squamous cell carcinoma (SCC) is slowly decreasing. Beyond this epidemiologic change, the two histological subtypes differ in a number of features including risks factors, tumor location, tumor biology and outcomes (1).
In acknowledgement of these differences, the newest 8th edition of the American Joint Committee on Cancer (AJCC) staging of epithelial cancers of the esophagus and EGJ, which is scheduled to go into effect in the United States at the beginning of 2018, has refined this histology-specific disease stage with incorporation of new anatomic and non-anatomic descriptors (2-5). This reappraisal was justified by the fact that in the past (7th edition and previous editions), decision making and prognostication have been based on the pathological findings of the resected specimen after esophagectomy (6). Then, assignment of clinical stage grouping (cTNM) was made with the same prognosticator as used for pathologic stage grouping (pTNM). Thus, these inadequacies between clinical and pathological stage grouping have pointed out four shortcomings: (I) whether prognostic significance of cTNM is shared with pTNM remains controversial; (II) the pathological stage was inadequate for initial therapeutic decisions, which remain based on clinical findings before any treatment; (III) patients who did not received surgery were not consider; (IV) patients who received neoadjuvant treatment were included in the same stage grouping and the post-therapeutic effect was not considered.
For these reasons, the new 8th edition considers separate and temporally related cancer classification based on the treatment strategy: clinical cTNM (before any treatment), pathologic pTNM (after surgery alone) and postneoadjuvant pathologic ypTNM (after neoadjuvant treatment followed by surgery). Based on data of patients collected through the Worldwide Esophageal Cancer Collaboration (WECC) group (7-10), the 8th edition permits a more robust and reliable random forest–based machine learning analysis. Moreover, this new edition seems more adaptable to the current practice including neoadjuvant regimen.
The main objective of this review is to examine the current staging of esophageal cancer and the new aspects of the 8th edition with its applications to clinical practice.
The Worldwide Esophageal Cancer Collaboration (WECC) group in 2018
In 2002, the 6th edition of AJCC-UICC staging system for esophageal cancer was neither data-driven nor harmonized with the staging of stomach cancer. At the request of the AJCC, the WECC was created with the purpose to assemble multicenter international data from five countries and three continents. Established in 2009 at the initiative of T. W. Rice and E. Blackstone, WECC data were used for preparation of the 7th edition staging manuals (6,7). At this time, WECC encompassed 13 institutions, over 3 continents and summarizing 4,627 patients. In contrast to the 8th edition, the 7th edition was based on pathologic staging of tumors of patients undergoing esophageal resection alone (6,7). For the first time, analyses were performed on the correlation between cancer characteristics (anatomic and non-anatomic) and survival in order to generate stage groupings for which survival was: (I) monotonic (meaning that survival decreases with increasing of stage group), (II) distinctive between groups, and (III) homogeneous within groups with the same prognostic range.
In order to increase the number of patients, the numbers of variables collected, and to include patients who received neoadjuvant treatment, a six-continent WECC invitation was promoted in 2012 over 79 institutions aiming at constructing refined data-driven esophageal cancer staging for the 8th edition of the cancer staging manuals (8-10). Of these, 33 institutions submitted their data by September 30th, 2014. A total number of 22,654 patients were collected with esophageal cancers. Among them, 22,123 had clinical staging data available before treatment (SCC in 8,156, adenocarcinoma in 13,814, adenosquamous carcinoma in 116, and undifferentiated carcinoma in 37). These patients were assessed to draw the cTNM stage grouping (3,8). Among them, 13,300 patients had pathologic staging after esophagectomy or endoscopic resection or ablation alone. These patients constituted the cohort for pTNM stage grouping (4,9). The remaining 7,773 patients had pathologic staging data after neoadjuvant therapy and constituted the cohort for ypTNM stage grouping (5,10). The 8th edition was scheduled to go into effect in United States on January 1st 2018. The future of WECC will be to build on achievements of the 8th edition, to continue data driven staging based on WECC and to promote collaboration in order to prepare the next 9th revision of esophageal cancer staging with the specific goals to make simplification and to reconcile gastric and esophageal cancer.
Cancer categories and subcategories of the 8th edition
All the elements and cancer characteristics used for stage disease are now termed categories and subcategories. Current cancer staging categories of the 8th edition are reported in Table 1. Main changes between 7th and 8th edition are reported in Table 2. Anatomic categories include T descriptors (tumor invasion), N descriptors (regional lymph node invasion) and M descriptors (distant site). Non-anatomic categories include grade differentiation (G descriptors) and tumor location (L descriptors).

Full table

Full table
Anatomic categories
For T descriptors, subcategorization of pT1 into pT1a and pT1b enhanced and improved stage I grouping. Also for T2 SCC, the location of the tumor is removed as staging category. Meanwhile, subcategorization of pT4 in pT4a and pT4b, provides a much more reliable description of an advanced localized tumor invading adjacent structures with doubtful resectability. With this new classification, surgeons have the opportunity to make distinction between a resectable (T4a) and a non-resectable tumor (T4b). Now, T4a tumor includes direct invasion of peritoneum.
For N categories, regional lymph node mapping has been refined. In fact, the 7th edition lymph node mapping included station common with lung cancer staging system. As a result, some station were not regional nodes. The new map is reported in Table 3. Regional mapping is defined as any nodes found in the adventitia or periesophageal tissue from the upper esophageal sphincter to the celiac artery. Regional lymph node are now grouped in 18 stations. Regional lymph node includes whatever the histological subtype supraclavicular nodes (station 1L and 1R) and celiac nodes (station 20).
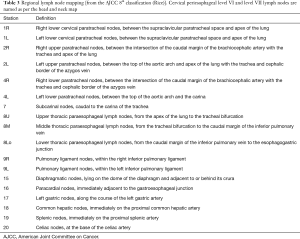
Full table
For M category, there are no change. Distant metastases are simply designated M0 (no distant metastasis) and M1 (distant metastasis). Subclassification M1a, M1b and MX are no longer used.
Non-anatomic categories
Grade differentiation (G categories) remains an important parameter for pathologic staging of early-stage cancer. As an example, undifferentiated tumors require further analysis to clearly state the cell type origin: if a glandular origin is identified, the tumor will be staged as G3 adenocarcinoma; if a squamous origin is determined or if the tumor remains undifferentiated, a G3 SCC will be considered.
At last, the location of the tumor (L categories) affects stage grouping. The tumor location includes typical endoscopic measurements of each region measured from the incisors. Exact measurements depend on body size and height. Location of cancer primary site is defined by cancer epicenter. Cancers involving the EGJ that have their epicenter within the proximal 2 cm of the cardia (Siewert types I/II) are to be staged as esophageal cancers. Cancers with epicenter more than 2 cm distal from the EGJ, even if the EGJ is involved, will be staged using the stomach cancer TNM and stage groups. For SCC, tumor location in combination with grade will be required for subgrouping pT3N0M0.
Cancer stage grouping of the 8th edition
The new 8th edition for cancer of the esophagus and EGJ is data driven and considers separate and temporally related cancer classification based on the treatment strategy: prior to any treatment (clinical stage cTNM); after surgical resection alone or after endoscopic resection (pathological stage pTNM); after multimodal treatment prior to surgery (ycTNM); after multimodal treatment after surgery (ypTNM); at time of recurrence (retreatment stage rTNM); and death (autopsy stage aTNM). Moreover, the 8th edition considers histological subtype in stage grouping, making distinction between adenocarcinoma and SCC.
cTNM
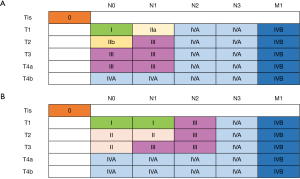
New to the 8th edition, cTNM is prior to treatment decision. Dissimilar stage group composition and survival profiles necessitated cTNM distinct from pTNM. There is separate clinical staging for adenocarcinoma and SCC (Figure 1). Clinical staging is chiefly based on imaging and thus is limited by resolution of each individual technique. Standardization of the methods of staging is necessary. Every effort should be made to prove invasion of regional or distant lymph nodes. Because lymph node invasion (cN1) is considered to be a high-risk finding for both squamous cell and adenocarcinoma, histologic proof of cN+ should be the rule, even if it should lead to reconsideration of clinical stage and treatment strategy.
pTNM
Historically, 8th edition expanded from the 7th edition based only on pTNM. Pathologic staging considers tumor classification after surgery alone or after endoscopic mucosal resection. Pathologic stage grouping can neither direct pre-treatment decisions nor aid in prognostication for treatment other than esophagectomy or endoscopic treatment. For these reasons, pTNM has lost some of its clinical relevance for advanced stage cancer as neoadjuvant therapy replaces esophagectomy alone. However, it remains important for early-stage cancers where surgery stands the first line treatment. Dissimilar stage group composition and survival profiles necessitated separate staging groupings for adenocarcinoma and SCC (Figure 2). Location category is used only to stage pT3N0M0G2-3 for SCC. Stage IIB is an interesting group wherein “T meets N”. For this subcategory, survival of the uncommon superficial pT1 cancers with one or two regional nodal metastases is similar to the more common, deeply invasive pT3 cancers free of regional nodal metastases.

Neoadjuvant pathologic stage grouping (ypTNM)
The proportion of patients receiving neoadjuvant therapy is increasing worldwide for esophageal cancer and EGJ. However, patients undergoing neoadjuvant therapy (ypTNM) and those receiving esophagectomy alone (pTNM) cannot share stage groups because of the random response to neoadjuvant therapy. New to the 8th edition is stage grouping of patients with esophageal cancers who have had neoadjuvant therapy and pathologic review of the resection specimen (ypTNM) (Figure 3). Drivers of this addition include absence of equivalent pathologic (pTNM) categories for the peculiar neoadjuvant pathologic categories (ypT0N0-3M0 and ypTisN0-3M0), divergent stage group compositions, and markedly different survival profiles. Grade and location play no role in neoadjuvant pathologic stage grouping. The groupings are identical for both histopathologic cell types. Prognostication is possible, but survival is reduced from what has been classically quoted for early and intermediate pTNM stage groups. Persistent regional lymph node metastases (ypN1) portend poor survival, and sterilization of metastatic regional lymph nodes (ypN0) does not equate with cure. Patients with ypN0 cancers confined to the esophageal wall or those with complete response have an intermediate survival regardless of ypT.
Recurrent cancer staging (rTNM) and autopsy staging (aTNM)
Recurrence of the disease after a first-line treatment and after a cancer-free interval is termed rTNM. This situation can be observed after an interval of months or years after surgery alone, after neoadjuvant treatment followed by surgery or after definitive (exclusive) chemoradiotherapy. When surgery is proposed in this last option, the surgery is termed “salvage esophagectomy”.
The autopsy staging (aTNM) is when a post-mortem examination determines the stage of the disease. The aTNM can be determined in known and treated patients. It can be determined in untreated patients. The natural history of the disease and the associated risk factors can provide useful information in epidemiologic studies.
Clinical determination of anatomical and non-anatomical categories
The clinical assessment of anatomical (T, N, M descriptors) and non-anatomical (histological grade and location) characteristics is obtained using esophagoscopy with biopsy, endoscopy ultrasound fine needle aspiration biopsy (EUS-FNA), thoracic-abdominal-pelvic CT and whole body FDG-PET fused with CT. This clinical assessment provides the baseline for evaluation of therapeutic options and rational treatment decisions. If necessary, this baseline preoperative workup may be supplemented by cervical lymph node biopsy, mediastinoscopy, thoracoscopy, laparoscopy, bronchoscopy, endoscopic bronchial ultrasound (EBUS) and CT-directed percutaneous biopsy.
Determination of clinical TNM descriptors
Determination of cT is mainly based on EUS. Invasion of the muscularis mucosae makes distinction between T1a and T1b. Invasion of muscularis propria, but confined to this layer, indicates a cT2. Invasion beyond the muscularis propria defines cT3. When adjacent structures are invaded, cT4 is considered. Accuracy of EUS for cT determination is around 80%, with the best accuracy for T3–T4 tumors (11-13). Unfortunately, EUS has its own limitation and a malignant stricture prohibiting passage of the probe is highly predictive of an advanced stage and should be considered at least as a T3 tumor. A tumor length >5 cm is also predictive of T3 tumor (14). Determination of cT could be supplemented by CT in case of cT3 with malignant stricture or in case of cT4 tumor to rule out invasion of adjacent structures with preservation of fat planes. At last, cT determination can be improved with the pathological result of an endoscopic mucosal resection. In this case, staging is in reality a pT (analysis of the resected mucosa) making distinction between T1a and T1b.
Determination of cN is again based mainly on EUS-FNA. EUS alone is only 20% specific for detecting N+ disease resulting in overstating in 80% of pN0 patients (15). Criteria of malignancy on EUS are node size >5 mm, round borders, a smooth shape and hypoechoic center. The diagnostic performance of EUS-FNA in the determination of cN is 92% sensitivity, 93% specificity, 100% positive predictive value and 86% negative predictive value (16). Determination of cN can be supplemented by others investigations. An enlarged lymph node on CT suggest nodal metastasis. Intrathoracic and abdominal lymph nodes with a short axis >1 cm are considered enlarged and a short axis >0.6 cm is considered pathological for supraclavicular and cervical lymph node. Accuracy of FDG-PET in determining N status is highly variable, ranging from 37% to 90% (17). In a large meta-analysis, FDG-PET is of 57% (range, 43–70%) sensitive and 85% (range, 76–95%) specific (18).
Determination of cM is based on investigations able to rule out metastasis according to their privileged sites: liver in 35%, lung in 20% and adrenal gland in 2%. Current investigations include routine thoracic-abdominal-pelvic CT. Brain CT is not regularly performed because of the low rate of brain metastasis (less 1%). Whole body FDG-PET fused with CT (PET-CT) is nowadays recommended. Accuracy of PET-CT seems to be excellent to rule out metastasis (88%). Positive and negative predictive values are 68% and 99%, respectively, for classifying cM (19). As a result, integration of whole body PET-CT to clinical stage led to stage and treatment being revised in 34% and 26% of patients, respectively. Moreover, with PET-CT, synchronous cancer is detected in near 2%. At last, determination of cM can be supplemented by exploratory laparoscopy or thoracoscopy. Laparoscopy is known to potentially change the treatment strategy in 10% of patients, allowing resection in 2% who were initially overstated and avoiding resection in 8% due to unforeseen peritoneal or liver metastases (20).
Determination of neoadjuvant clinical stage (ycTNM): restaging after multimodal therapy
Another area where clinical assessment of disease stage is somewhat challenging is the restaging after initial induction therapy, a therapeutic modality that is increasingly being used in patients with locally advanced disease. In this situation, determinations of ycT and ycN are problematic. Thoracic-abdominal-pelvic CT is systematically ordered for morphological assessment of the mediastinum. Endoscopy with biopsies is decisive to assess potential residual disease and to allow distinction between responders and non-responders to induction therapy. EUS does not allow an accurate determination of ycT and ycN after both chemo and combined chemoradiotherapy as it is not able to make distinction between cancer, post-treatment necrosis, fibrosis or inflammation. Accuracy of EUS in this situation is only 27% to 59% (21-24). In contrast, determination of ycM can be obtained by whole-body PET/CT as this investigation is able to detect distant metastases in approximately 8% of patients following induction chemoradiotherapy (25). Some oncologists routinely incorporate a post-induction-therapy PET/CT approximately four weeks after the completion of induction therapy to determine the ycM . In addition, post-induction-therapy FDG-PET offers information on metabolic response to induction therapy. Some series suggest that metabolic responders had a significantly better prognosis than did non-responders (26-28). However, PET-directed therapy cannot yet be considered a standard approach.
Determination of pathological (pTNM) or neoadjuvant pathological stage (ypTNM)
The pathological stage is obtained from the definitive analysis of the resected specimen: after surgery alone pTNM or after neoadjuvant treatment (ypTNM). Even if recommendations are available for the handling of resected specimens (29), there are no standardized criteria for their examination. . Sections are taken at the proximal and distal resection margins, the esophagus proximal and distal to the tumor and the EGJ. It is largely accepted to analyze 8 to 11 sections. The tumor should have its soft tissue margin marked with ink and be sectioned at its largest point. The superior and inferior boundaries of the tumor should be sectioned. All lymph nodes should be dissected from the resected specimen and be evaluated with the lymphadenectomy specimen. There is a general agreement, when possible, for surgeons to send lymph node specimens, for analysis separately from the resected specimen. The surgeon and the pathologist must work together to avoid a gap between the number of resected lymph nodes and the number of those analyzed. Lymph nodes are typically analyzed by using a single representative section from each individual lymph node. Pathological assessment requires the removal of sufficient lymph nodes in order to evaluate the pN and the ypN category. Because the highest N classification (N3) is ≥7 positives nodes, any resection should theoretically include at least 7 resected lymph nodes to be correctly interpreted. The recommendations, adopted by AJCC, are to resect a minimum of 10 lymph nodes for T1 cancers, 20 for T2 cancers and 30 for T3 cancers (7).
Although the impact of proximal and distal resection margin is well known, the definition of the circumferential (radial) margin (CRM) continues to be debated (30). There are two definitions of positive CRM. The UK Royal College of Pathologists considers the CRM to be positive if the tumor is found within 1 mm of the surgical margin, whereas the College of American Pathologists (CAP) defines a positive CRM as tumor found at the cut margin of resection. Whatever the treatment strategy (surgery alone or after neoadjuvant treatment), definition of CRM should be based on CAP definition (30,31).
At last, after neoadjuvant treatment, tumor regression grade (TRG) has to be evaluated. Mandard grading can be applied even if its application in ypTNM determination is not well established. Mandard grading may assess TRG according to the amount of fibrosis in relation to residual tumor cells (32). Significant regressive changes may result in complete disappearance of malignant cells and replacement of the tumor by fibrous or fibro-inflammatory granulation tissue. The prognostic value of TRG may even exceed those of currently used staging systems. However, there are some limitations regarding interobserver variability, especially in borderline cases, which may be improved by standardization of the work-up of resection specimens and better training of histopathologic determination of regressive changes (33).
Determination of recurrent cancer staging
Determination of local cancer recurrence (rT) can be established using endoscopy and biopsy or by EUS-FNA, especially after definitive chemoradiotherapy because of the huge fibrosis into the esophageal wall after completion of full-dose radiotherapy. Endoscopic surveillance of the gastric tube and the esophago-gastric anastomosis should be regular and guided by any new symptoms of dysphagia. Surveillance is normally based on 5-year thoracic-abdominal-pelvic CT program (every 6 months during the first 2 years and every year for the last 3 years). Determination of regional (rN) or distant (rM) recurrences can be obtained by any morphological investigations. The diagnosis yield of PET-FDG is 100% sensitivity, 85% specificity, and 100% positive predictive value. Documentation of recurrence is essential and may include any available techniques (mediastinoscopy, thoracoscopy, laparoscopy, CT-guided biopsy).
Conclusions and perspectives
The 8th edition of staging of cancer of the esophagus and EGJ is data driven and expanded from the 7th edition of pTNM only, to include pathologic stage groups after neoadjuvant therapy (ypTNM) and clinical stage groups (cTNM) before treatment decision. This latest TNM edition is a histology-specific prognostic stage groupings created to acknowledge the dramatic change in epidemiology of esophageal cancer and EGJ observed since the last decade. Refinement of each T, N, M categories and subcategories makes the 8th edition more reliable and more adaptable to the current practice including neoadjuvant regimen. Regional lymph node mapping has been refined. Stage grouping in its three components (c, p or ypTNM) mixed with grade, histology and location should be seen as a complex stratification. It must be remembered that what is important for stage grouping of populations is not necessarily helpful for the individual patient.
Critical evaluation of 8th edition staging, intensive data collection, in-depth analyses, and further consensus appraisal are necessary to proceed from the 8th to the 9th edition. The proposal to develop a current online data registry would be the best way to have the entire data collection. One of the criticisms against WECC database is its granularity, and not having all the data fields filled in meant that the data were not included and, hence, contributions were lost. Many efforts should be made to provide with the best quality data the upcoming 9th definition, scheduled to be published in 2024.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers 2017;3:17048. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol 2017;12:36-42.
- Rice TW, Ishwaran H, Blackstone EH, et al. Recommendations for clinical staging (cTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus 2016;29:913-19.
- Rice TW, Ishwaran H, Hofstetter WL, et al. Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus 2016;29:897-905.
- Rice TW, Ishwaran H, Kelsen DP, et al. Recommendations for neoadjuvant pathologic staging (ypTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus 2016;29:906-12.
- Rice T W, Rusch V W, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction; data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Staging Manuals. Cancer 2010;116:3763-73.
- Rice T W, Rusch V W, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1-8. [Crossref] [PubMed]
- Rice TW, Apperson-Hansen C, DiPaola LM, et al. Worldwide Esophageal Cancer Collaboration. clinical staging data. Dis Esophagus 2016;29:707-714. [Crossref] [PubMed]
- Rice TW, Chen LQ, Hofstetter WL, et al. Worldwide Esophageal Cancer Collaboration. pathologic staging data. Dis Esophagus 2016;29:724-733. [Crossref] [PubMed]
- Rice TW, Lerut TE, Orringer MB, et al. Worldwide Esophageal Cancer Collaboration. neoadjuvant pathologic staging data. Dis Esophagus 2016;29:715-723. [Crossref] [PubMed]
- Lightdale CJ, Kulkarni KG. Role of endoscopic ultrasonography in the staging and follow-up of esophageal cancer. J Clin Oncol 2005;23:4483-9. [Crossref] [PubMed]
- Rice TW, Blackstone EH, Adelstein DJ, et al. Role of clinically determined depth of tumor invasion in the treatment of esophageal carcinoma. J Thorac Cardiovasc Surg 2003;125:1091-102. [Crossref] [PubMed]
- Heidemann J, Schilling MK, Schmassmann A, et al. Accuracy of endoscopic ultrasonography in preoperative staging of esophageal carcinoma. Dig Surg 2000;17:219-24. [Crossref] [PubMed]
- Bhutani MS, Barde CJ, Markert RJ, et al. Length of esophageal cancer and degree of luminal stenosis during upper endoscopy predict T stage by endoscopic ultrasound. Endoscopy 2002;34:461-3. [Crossref] [PubMed]
- Kutup A, Link BC, Schurr PG, Strate T, et al. Quality control of endoscopic ultrasound in preoperative staging of esophageal cancer. Endoscopy 2007;39:715-9. [Crossref] [PubMed]
- Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 1997;112:1087-95. [Crossref] [PubMed]
- Lerut T, Flamen P, Ectors N, et al. Histopathologic validation of lymph node staging with FDG-PET scan in cancer of the esophagus and gastroesophageal junction: A prospective study based on primary surgery with extensive lymphadenectomy. Ann Surg 2000;232:743-52. [Crossref] [PubMed]
- van Vliet EP, Heijenbrok-Kal MH, Hunink MG, et al. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer 2008;98:547-57. [Crossref] [PubMed]
- Noble F, Bailey D, et al. Impact of integrated PET/CT in the staging of oesophageal cancer: a UK population-based cohort study. Clin Radiol 2009;64:699-705. [Crossref] [PubMed]
- Bonavina L, Incarbone R, Lattuada E, et al. Preoperative laparoscopy in management of patients with carcinoma of the esophagus and of the esophagogastric junction. J Surg Oncol 1997;65:171-4. [Crossref] [PubMed]
- Beseth BD, Bedford R, Isacoff WH, et al. Endoscopic ultrasound does not accurately assess pathologic stage of esophageal cancer after neoadjuvant chemoradiotherapy. Am Surg 2000;66:827-31. [PubMed]
- Isenberg G, Chak A, Canto MI, et al. Endoscopic ultrasound in restaging of esophageal cancer after neoadjuvant chemoradiation. Gastrointest Endosc 1998;48:158-63. [Crossref] [PubMed]
- Griffin JM, Reed CE, Denlinger CE. Utility of restaging endoscopic ultrasound after neoadjuvant therapy for esophageal cancer. Ann Thorac Surg 2012;93:1855-9. [Crossref] [PubMed]
- Kalha I, Kaw M, Fukami N, et al. The accuracy of endoscopic ultrasound for restaging esophageal carcinoma after chemoradiation therapy. Cancer 2004;101:940-7. [Crossref] [PubMed]
- Bruzzi JF, Munden RF, Truong MT, et al. PET/CT of esophageal cancer: its role in clinical management. Radiographics 2007;27:1635-52. [Crossref] [PubMed]
- Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 2007;8:797-805. [Crossref] [PubMed]
- Javeri H, Xiao L, Rohren E, et al. Influence of the baseline 18F-fluoro-2-deoxy-D-glucose positron emission tomography results on survival and pathologic response in patients with gastroesophageal cancer undergoing chemoradiation. Cancer 2009;115:624-30. [Crossref] [PubMed]
- Ott K, Herrmann K, Krause BJ, et al. The Value of PET Imaging in Patients with Localized Gastroesophageal Cancer. Gastrointest Cancer Res 2008;2:287-94. [PubMed]
- Haggitt RC, Appelman HD, Lewin KJ, et al. Recommendations for the reporting of resected esophageal carcinomas. Association of Directors of Anatomic Surgical Pathology. Hum Pathol 2000;31:1188-90. [Crossref] [PubMed]
- Chan DS, Reid TD, Howell I, et al. Systematic review and meta-analysis of the influence of circumferential resection margin involvement on survival in patients with operable oesophageal cancer. Br J Surg 2013;100:456-64. [Crossref] [PubMed]
- Depypere L, Moons J, Lerut T, et al. Prognostic value of the circumferential resection margin and its definitions in esophageal cancer patients after neoadjuvant chemoradiotherapy. Dis Esophagus 2018.31. [PubMed]
- Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [Crossref] [PubMed]
- Thies S, Langer R. Tumor regression grading of gastrointestinal carcinomas after neoadjuvant treatment. Front Oncol 2013;3:262. [Crossref] [PubMed]



