Successful establishment of a left ventricular assist device program in an emerging country: one year experience
Introduction
The first open –heart surgery in Serbia was performed in 1946 at the Clinical Center of Serbia, the first cardiac surgery center in the country. Today, there are five cardiac surgery centers across the country with around 7 million residents. The heart transplant (HT) programme started in 1995 but lasted only 4 years because of the socio-economic and war situations in Serbia. At the end of 2013 in the Clinical Center of Serbia the HT program started once again and left ventricular assist devices (LVAD) program was also introduced. Due to insufficient availability of donor hearts only 23 HT were done till now. A similar situation is present in more developed country, but the economic situation in those countries provides a well-established LVAD program for all their residents (1). Despite the still present poor economic situation in the country LVAD program continued to develop as the only alternative for HT for this group of patients. For the entire country, the Clinical Center of Serbia is the only center that provides LVAD support for patients with end-stage heart failure (HF) awaiting HT, although for most of them it will be the definitive way of treatment (DT). The latest generation of LVAD, continuous flow left ventricular assist devices (CF-LVAD), has played a significant role in reducing mortality in patients who are on the transplant list, and has become a standard procedure as a bridge to transplantation (BTT) (2-4). With the current CF-LVAD, survival rate is much better; 1-year survival for BTT and DT is 80% and 76%, respectively. Despite the fact that the overall adverse events with new technology of devices are significantly lower, only around 30% of patients are free from any major adverse event at 1 year (5). Taking into consideration that only approximately one third of adult LVAD patients receive a HT by 1 year, and that number is significantly lower in less developed countries meaning that dealing with side effects is much longer, it is very important to define the predictors of survival for these patients. The primary goal of this study was to evaluate end-stage HF patient outcomes after CF-LVAD implantation in a developing country and to compare to those reported by centers in developed countries. The secondary goal was to determine factors that may be connected to improved survival in this group of patients.
Methods
Studied population
This is a prospective study of 47 consecutive patients with end-stage HF [indicated by Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) score 2–5] who received CF-LVAD at our institution between June 2013 and January 2016. Exclusion criteria included severe pulmonary or hepatic dysfunction, renal failure, active infection, and aortic aneurysm. In 42 patients CF-LVAD was used as BTT and in 5 patients as DT. The HeartMate II (Abbott, Chicago, IL, USA) was implanted in 35 (75%) patients and HVAD (Medtronic, Minnesota, MA, USA) in 12 (26%) patients.
Follow up
After CF-LVAD implantation, a standardized anticoagulation regimen was used with initiation of infusion of heparin followed by transition to warfarin as well as aspirin. Postoperative medical management, including inotropic, antiarrhythmic and HF therapy was performed according to usual practice. Functional, cardiac [echocardiographic and brain-type natriuretic peptide (BNP)], renal and liver function assessments were obtained at baseline, before LVAD implantation and at 3, 6, 12 months. After 1 year both the overall survival and adverse event profiles were evaluated. According to the New York Heart Association (NYHA) criteria the patient functional status was assessed. Left ventricular (LV) diameters and left ventricular ejection fraction (LVEF) were measured using two-dimensional transthoracic echocardiography (TTE), which was performed in a standard manner using Vivid E 9 (GE Medical Systems, Milwaukee, WI). Both left ventricular end-systolic (LVESD) and end-diastolic (LVEDD) diameters were measured from two-dimensional echocardiographic images in the parasternal long-axis view and M-mode echocardiography. The LVEF was calculated using the Teichholz method and Simpson method (6). Renal function was assessed according to serum creatinine (µmol/L), blood urea nitrogen (BUN) (mmol/L) and estimated glomerular filtration rate [eGFR (mL/min/1.73 m2)]. eGFR was calculated based on the results of blood creatinine, age, sex and race. Patients with eGFR greater than 60 mL/min/1.73 m2 were defined as having normal renal function, and patients with eGFR less than 60 mL/min/1.73 m2 were defined as having renal dysfunction (RD). Among those with RD, patients were classified as moderate RD with eGFR 30–59 mL/min/1.73 m2, severe RD with eGFR 15–29 mL/min/1.73 m2 and renal failure (RF) with eGFR less than 15 mL/min/1.73 m2. Hepatic function was assessed based on measurement level of aspartate transaminase [AST (IU/L)], alanine transaminase [ALT (IU/L)] and total bilirubin (µmol/L). Presence of adverse events during CF-LVAD support including any kind of infection [devices related infection: driveline (DL) and pump pocket infections or non-devices related infection], thrombosis of CF-LVAD, right ventricular (RV) failure (requiring prolonged inotropes support or RV assist device (RVAD) support), stroke (hemorrhagic or ischemic), bleeding, RD, RF, aortic insufficiency (AI) and multiorgan failure (MOF). Bleeding considered requiring more than two units of packed red blood cells (PRBCs) per 24-hour period after CF-LVAD implantation, bleeding requiring reoperation and gastrointestinal bleeding (GIB). GIB was defined as overt gastrointestinal tract bleeding (upper or lower sections) or presence of blood in the stool confirmed by Hemoccult and reduced hemoglobin levels by more than or equal to 10 g/L with no other reason for anemia. After CF-LVAD implantation, survival was assessed at three specific time points: 30 days, 6 months and 1 year. Early mortality was defined as death within 30 days of surgery or before hospital discharge.
Statistical analysis
Quantitative variables are expressed as mean values with standard deviations or as medians with interquartile ranges. Categorical data are presented by absolute numbers with percentages. Kolmogorov-Smirnov test was used to assess the data distribution. Changes in examined variables from baseline to the 3, 6 and 12 months of LVAD use were evaluated by Repeated Measures ANOVA or Friedman test. The overall survival was estimated by the Kaplan Meier survival analysis and was defined as time from LVAD implantation to death. For patients who are event free, the censoring time is calculated as a time interval between date of LVAD implantation and the patient’s final contact with available data concerning the event (the last follow-up date or HT date). The estimates and graphical presentation are performed via Kaplan Meier approach. To identify predictors of overall survival univariate and multivariate Cox regression analysis was used. Occurrence of adverse events is calculated per patient in the first year of LVAD support. Statistical analysis was performed using SPSS statistical software (SPSS for Windows, release 21.0, SPSS, Chicago, IL, USA). In all tests, P value <0.05 was considered to be statistically significant.
Results
Patient characteristics
Baseline demographic and preoperative characteristics of CF-LVAD recipients are given in Table 1. The youngest patient was 18 years old at the time of LVAD procedure and the oldest one 73 years old. 94% of the subjects were male. Ischemic cardiomyopathy was the most frequent HF etiology (53%). Fifty-five percent of the subjected were indicated with INTERMACS profile 4. Preoperative hemodynamic and intraoperative data are shown in Table 2.
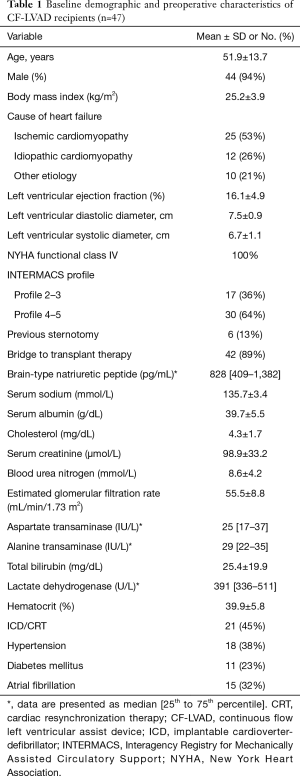
Full table
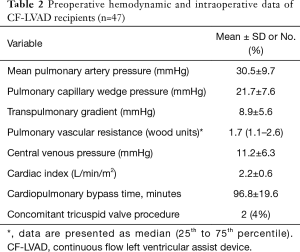
Full table
Outcomes
Following implant surgery, the median time in the intensive care unit was 10.5 days (5–15 days), and the median duration of total hospitalization was 23 days (17–30 days). The median duration of CF-LVAD support was 13 months (12–21 months). As our center has nascent HT program, only 4 of 42 CF-LVAD patients received HT, after a median duration of CF-LVAD support of 13.5 months (13–16.3 months).
Survival analysis
For both BTT and DT CF-LVAD patients the 30 days, 6 months and 1 year survival rates were 89%, 85% and 80%, respectively. In the first year MOF was the most frequent cause of death (n=4; all died within the first 30 days of implantation). Other causes of death were pump thrombosis, infection, bleeding and hemorrhagic stroke.
Cardiac, functional and end-organ function after CF-LVAD
Cardiac (echocardiographic parameters and BNP), functional and end-organ function changes at baseline, 3 months, 6 months and at 1 year in CF-LVAD recipients are presented in Table 3. At 3 months post-CF-LVAD implantation, a significant improvement was observed in LV (EDD, ESD) dimensions from baseline values of 7.5±0.9 and 6.7±1.1; to 6.2±1.0 and 5.3±1.2; respectively (P<0.001). For LVEF and BNP, the baseline values of 16.1±4.9 and 828 [409–1,382], improved to 29.7±10.7 and 304 [145–597], respectively (P<0.001). This improvement for all above mentioned parameters was sustained over the time of follow-up. NYHA functional classification significantly improved 3 months after CF-LVAD implantation from 4.0 at baseline to 2.1±0.5 (P<0.001), continued to improve till 6 months to 1.6±0.5 (P<0.001) and was stable throughout the entire first year follow up.
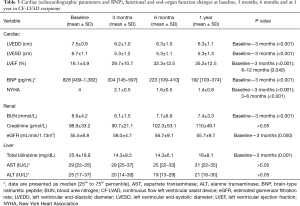
Full table
In all patients who underwent CF-LVAD (n=47) there were slight trends towards improvement in eGFR for the period of first 3 months (55.5±8.8 vs. 58.0±4.7, P=0.050) and there was no change in serum creatinine for the whole follow-up period (98.9±33.2 vs. 110±49.1, P=0.067). In the patient group with baseline RD (eGFR <60 mL/min/1.73 m2), renal function showed significant improvements of eGFR from baseline values of 44.4±9.8 to 3 months values of 54.7±6.4 (P=0.004), with no follow up changes. We found significant reduction in BUN level for overall cohort, with significant decrease at 3 months of follow-up (8.6±4.2 vs. 6.1±1.5, P<0.001), and then remained in the normal range. With regard to hepatic function, in all cohorts of patients, total bilirubin showed a significant reduction throughout the first year of follow-up (P=0.001), with much more reduction in the first 3 months (25.4±19.9 vs. 14.3±8.5, P=0.001), which was sustained over the whole study period. There were no significant changes of AST and ALT values during the whole follow-up period (P=0.726 and P=0.085, respectively).
Adverse events
Adverse events during 1-year of CF-LVAD support are reported in Table 4. Adverse events were present in 27 patients (57.4%). The most common complication was bleeding (40%) which presented as either GIB (15%), or postoperative bleeding requiring reoperation (30%) and was followed by infection of any cause (21%). Patients with infection were treated with prolonged treatment of antibiotics based on culture results with low threshold to treat. Two of 3 patients with DL infection successfully underwent DL relocation on the contralateral side with an intra-abdominal route. Regarding pump thrombosis (9%), all patients were treated with fibrinolytic therapy (Actylisa), out of which one with success while 3 patients died, because of hemorrhagic stroke after fibrinolytic therapy. Other patients with stroke were treated with medication successfully. No patients underwent pump exchanging or explantation.
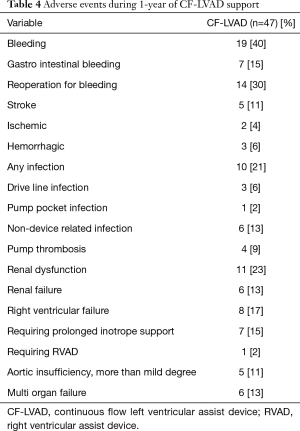
Full table
Predictors of survival
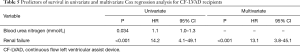
Among the entire cohort, in univariate analysis, BUN and RF developed within the first 30 days after implantation have been shown as significant predictors of survival (HR =1.1, P=0.034 and HR =14.2, P<0.001, respectively). In multivariate Cox regression analysis RF was found to be an independent risk factor for the overall survival (HR =13.1, P<0.001) (Table 5).
Full table
Discussion
Our study shows that patients treated with CF-LVAD, have a significant improvement in the functional status based on NYHA functional class, dimensions of LV, LVEF, BNP, total bilirubin and BUN, while improvement in creatinine and eGFR were present in patients with baseline impairment of renal function. We found that the RF developed within the first 30 days after implantation, was an independent risk factor for overall survival.
In recent years, with improvements in pump technology, considerable progress has been made to improve the outcomes of patients who undergo LVAD surgery, both as BTT and DT. An opinion has been established that CF-LVAD have improved the survival rate, hemodynamics, end-organ function and functional capacity of patients with end-stage HF awaiting HT or those who underwent LVAD as DT therapy (7-10). The 1-year survival rate for BTT indication showed significant improvement in contrast to early stage of clinical trials (68% vs. 80%) (5,9), the same as for DT indication (68% vs. 73%) (7,10). Our data extends previous findings and is comparable with those from the INTERMACS registry (5). Besides the obvious benefit of reducing mortality, many studies have shown the improvement in end-organ function recovery, after CF-LVAD implantation. Our study, among others, has shown significant improvement in both renal and liver function within 3 months of device implantation, especially in those with baseline abnormal end-organ function and remained normal throughout the whole follow-up period (11-14). The number of patients who recovered cardiac function after CF-LVAD implantation in terms LVAD explantation is very small, but number of CF-LVAD patients with getting adequate LV unloading and improving LVEF and LV dimensions are significantly higher. The significance of echocardiographic evidence of adequate unloading LV is closely connected with patient outcomes as a predictor of survival (15). The results of this study are consistent with previously published report by demonstrating a significant improvement in LV dimensions, LVEF and BNP values in patients who underwent implantation of CF-LVAD for the whole follow-up period (15). Besides the obvious improvement in survival rate, CF-LVAD significantly improved the functional status in these groups of patients, as our study has also confirmed (7-10). However, despite all of these benefits, there is still a significant incidence of device–related complications. Our study has shown adverse event rates, including bleeding, infection, stroke, pump thrombosis, AI, RV failure and RF, comparable with reports of the large clinical trials and results published by INTERMACS with bleeding as the most frequent adverse event (5,7-10).
In order to improve clinical outcomes after CF-LVAD implantation many studies have tried to identify predictors of post CF-LVAD survival (16-19). The key of LVAD success is to avoid end-organ dysfunction, so identification of these parameters can be useful for better patient selection for CF-LVAD procedure and better postoperative outcomes. In our study, we found that the RF developed within the first 30 days after implantation, was an independent risk factor for overall survival in multivariate analysis and suggests that clinicians should not to wait too long before making decision for CF-LVAD implantation. Bleeding is the most frequent complication extending total hospital stay, with resultant increased infection rate and further risk of bleeding and thrombotic events due to the need to change anticoagulation therapy. It is possible that using a novel implantation technique (upper hemisternotomy combined with anterolateral thoracotomy) as well as a new design of pumps may reduce complication rates (minimize trauma, reduce bleeding complication, infection complication and avoid RV failure (20-22). The latest generation of LVAD, the HeartMate 3 Left Ventricular Assist System, centrifugal-flow device with a fully levitated rotor, has shown much better short-term outcomes with lower adverse event rates (23-25). Further randomized studies should be performed in order to identify whether improved outcomes are sustained with longer follow-up periods on LVAD support as well as baseline cut-off level of end-organ function that can improve risk assessment of patients for LVAD therapy.
Limitations
We acknowledge some notable limitations in our study. The study was conducted in a single tertiary medical center; hence, there may be patient selection bias. Further investigation with a larger patient cohort may increase statistical significance and help conclusively identify other predictors of survival which may subsequently influence long term survival of this group of patients.
Conclusions
In conclusion, our data extends previous findings from centers of developed country, that CF-LVAD is an adequate treatment option for end-stage HF patients, and encourage expansion of CF-LVAD implantation in developing countries with nascent HT program. As renal failure is common following CF-LVAD implantation and is an independent risk factor for overall survival, the resolution of optimal timing of CF-LVAD implantation will contribute to maintaining favorable outcomes and better survival rate in this group of patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: JD Schmitto is consultant for Abbott and Medtronic. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our Institutional Review Board (approval number 29/VII15), in compliance with Helsinki Declaration (1996). Informed patient consent for data collection and analysis was obtained prior to the LVAD procedure.
References
- Pya Y, Bekbossynova M, Jetybayeva S, et al. Initial 3-year outcomes with left ventricular assist devices in a country with a nascent heart transplantation program. ESC Heart Fail 2016;3:26-34. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Kormos RL, et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant 2012;31:117-26. [Crossref] [PubMed]
- Patel CB, Alexander KM, Rogers JG. Mechanical circulatory support for advanced heart failure. Curr Treat Options Cardiovasc Med 2010;12:549-65. [Crossref] [PubMed]
- Rogers JG, Aeronson KD, Boyle AJ, et al. HeartMate II Investigators. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol 2010;55:1826-34. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015;34:1495-504. [Crossref] [PubMed]
- Lang RM, Bierig M, Devereux RB, et al. American Society of Echocardiography's Nomenclature and Standards Committee. Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification. Eur J Echocardiogr 2006;7:79-108. [Crossref] [PubMed]
- Slaughter MS, Rogers JG, Milano CA, et al. HeartMate II Investigators.Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. [Crossref] [PubMed]
- Pagani FD, Miller LW, Russell SD, et al. HeartMate II Investigators. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol 2009;54:312-21. [Crossref] [PubMed]
- Miller LW, Pagani FD, Russell SD, et al. HeartMate II Clinical Investigators. Use of a continuou - flow device in patients awaiting heart transplantation. N Engl J Med 2007;357:885-96. [Crossref] [PubMed]
- Park SJ, Milano CA, Tatooles AJ, et al. HeartMate II Clinical Investigators. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Heart Fail 2012;5:241-8. [Crossref] [PubMed]
- Sandner SE, Zimpfer D, Zrunek P, et al. Renal function and outcome after continuous flow left ventricular assist device implantation. Ann Thorac Surg 2009;87:1072-8. [Crossref] [PubMed]
- Hasin T, Topilsky Y, Schirger JA, et al. Changes in renal function after implantation of continuous-flow left ventricular assist devices. J Am Coll Cardiol 2012;59:26-36. [Crossref] [PubMed]
- Russell SD, Rogers JG, Milano CA, et al. HeartMate II Clinical Investigators.Renal and hepatic function improve in advanced heart failure patients during continuous-flow support with the HeartMate II left ventricular assist device. Circulation 2009;120:2352-7. [Crossref] [PubMed]
- Nadziakiewicz P, Szygula-Jurkiewicz B, Niklewski T, et al. Effects of Left Ventricular Assist Device Support on End-Organ Function in Patients With Heart Failure: Comparison of Pulsatile - and Continuous - Flow Support in a Single - Center Experience. Transplant Proc 2016;48:1775-80. [Crossref] [PubMed]
- Topilsky Y, Hasin T, Oh JK, Borgeson DD, et al. Echocardiographic variables after left ventricular assist device implantation associated with adverse outcome. Circ Cardiovasc Imaging 2011;4:648-61. [Crossref] [PubMed]
- Morgan JA, Go PH, Xuereb L, Kaur B, et al. Outcomes on Continuous Flow Left Ventricular Assist Devices: A Single Institutional 9-Year Experience. Ann Thorac Surg 2016;102:1266-73. [Crossref] [PubMed]
- Sandner SE, Zimpfer D, Zrunek P, et al. Age and outcome after continuous-flow left ventricular assist device implantation as bridge to transplantation. J Heart Lung Transplant 2009;28:367-72. [Crossref] [PubMed]
- Sandner SE, Zimpfer D, Zrunek P, et al. Renal function and outcome after continuous flow left ventricular assist device implantation. Ann Thorac Surg 2009;87:1072-8. [Crossref] [PubMed]
- Topkara VK, Coromilas EJ, Garan AR, et al. Preoperative Proteinuria and Reduced Glomerular Filtration Rate Predicts Renal Replacement Therapy in Patients Supported With Continuous-Flow Left Ventricular Assist Devices. Circ Heart Fail 2016;9. [Crossref] [PubMed]
- Schmitto JD, Molitoris U, Haverich A, et al. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg 2012;143:511-3. [Crossref] [PubMed]
- Schmitto JD, Hanke JS, Dogan G, et al. First Implantation of a Novel Left Ventricular Assist Device: The ReliantHeart aVAD. Ann Thorac Surg 2017;104:e311-e313. [Crossref] [PubMed]
- Hanke JS, Rojas SV, Avsar M, et al. Minimally-invasive LVAD Implantation: State of the Art. Curr Cardiol Rev 2015;11:246-51. [Crossref] [PubMed]
- Netuka I, Sood P, Pya Y, et al. Fully Magnetically Levitated Left Ventricular Assist System for Treating Advanced HF: A Multicenter Study. J Am Coll Cardiol 2015;66:2579-89. [Crossref] [PubMed]
- Schmitto JD, Hanke JS, Rojas SV, et al. First implantation in man of a new magnetically levitated left ventricular assist device (HeartMate III). J Heart Lung Transplant 2015;34:858-60. [Crossref] [PubMed]
- Hanke JS, Dogan G, Rojas SV, et al. First experiences with HeartMate 3 follow-up and adverse events. J Thorac Cardiovasc Surg 2017;154:173-8. [Crossref] [PubMed]


