Extended resections of large thymomas: importance of en bloc thymectomy
Introduction
Primary tumors of the thymus are infrequent, the most common histologic type is thymoma, a neoplasm that derives from thymic epithelial cells and constitutes the most frequent anterior mediastinal tumor in adults (1). The etiology of this tumor remains unknown, and due to its rarity, most research of thymoma have been based on single center reports. There is a peak incidence at 30–40 years of age for thymomas with myasthenia gravis (MG), and another peak around 60–70 years of age for those without MG. Women and men are affected with the same frequency. One third of patients are asymptomatic and will be referred due an incidental mediastinal mass on chest images, almost one third of thymoma patients will present MG and another third part will have local symptoms (2). Based on data of the Surveillance, Epidemiology, and End Results (SEER) database, thymomas in the United States show an incidence of 0.13 per 100,000 persons-years, this represents an estimate of just 390 cases per year in the whole country (3). There is no available data to estimate the incidence of this neoplasm in México. Thymomas comprise a spectrum of unique tumors with low to moderate malignant potential, according to the World Health Organization (WHO), thymomas are subdivided into types A, AB, B1, B2, B3 and C (1). These tumors have been associated with an indolent clinical course, nevertheless, all thymomas are malignant neoplasm, and have the ability to recur and to metastasize. Furthermore, the analysis of disease recurrence and overall survival (OS) can be challenging because of the long (nearly 10-year) disease-free intervals seen after thymectomy for thymomas (4).
The main objective of multi-modal treatment of thymomas should be complete surgical resection. Prognosis depends essentially on anatomical extent of tumor and the completeness of surgical resection (5). Large tumor size is not directly associated with survival, but is strongly associated with high rates of incomplete resections, which has a negative impact on the overall prognosis (4). In this work, we report our experience with thymomas larger than 5 cm.
Methods
An observational retrospective cohort study was conducted in patients with thymomas of 5 cm or larger, who underwent thymectomy from January 2005 to December 2016 at the Instituto Nacional de Cancerología in México. Data were collected from a database. All electronic and paper charts were reviewed by the main investigator. The study was approved by our Institutional Ethics Committee which waived the requirement of an individual patient consent because only non-sensible data were used for the analysis.
As institutional criteria on all patients with thymoma, the minimal resection is “complete thymectomy”. The limits of resection are both phrenic nerves, the inferior pole of the thyroid gland and the diaphragm; and should include the thymic gland, mediastinal fat and lymph nodes contained into the limits. Additionally, on cases in which there are firm adhesions to another structures, extended resections for an en bloc removal is the rule to provide negative margins. Completeness of resection was defined as complete resection (R0) when there is no evidence of residual tumor (macroscopically and/or microscopically), and incomplete resection when there is evidence of microscopically (R1) or macroscopically (R2) residual tumor.
Induction therapy is indicated on the tumor board session for patients considered as non-resectable by the thoracic surgery team, for patients with metastatic pleural disease or patients with type C thymoma. Indications for adjuvant therapy are based on pathological final report. Follow-up was performed after the end of treatment with clinical assessment and chest computed tomography (CT) every 4 months for the first 2 years, every 6 months for the next 3 years and then annually.
Primary end-points were rate of complete resection, morbidity and mortality of thymectomy. Secondary end-points were OS and disease-free survival (DFS). We report descriptive statistics as medians with ranges for continuous variables and frequencies and proportions for categorical variables. OS and DFS were calculated using the Kaplan-Meier method. DFS was defined as the time from the date of surgery to first recurrence (identified on CT scan). OS was defined as the time form surgery date to death from disease or last follow-up. Differences between survival curves were analyzed by the Log-rank test. We used Cox-proportional hazard regression models for multivariate analysis. Hazard ratios and 95% confidence intervals were estimated. Two-sided tests were applied and results were considered statistically significant if the P value was less than 0.05; SPSS v.22.0 software (SPSS Inc., Chicago, IL, USA) was used to perform the analysis.
Results
Pre-operative data
Twenty-five patients were identified and included in the final analysis, 15 (60%) were female. The mean age was 56.6 years (27–82 years). Thirteen patients (52%) did not present paraneoplastic syndrome. Median size of thymoma was 8.3 cm (5–14 cm). Three patients (12%) underwent induction treatment with chemotherapy; two cases of WHO C thymoma and one of B3 thymoma. In all cases, there was no certainty of resectability due to large size and invasion to neighborhood structures. Detailed demographic and preoperative characteristics of patients are shown in Table 1.
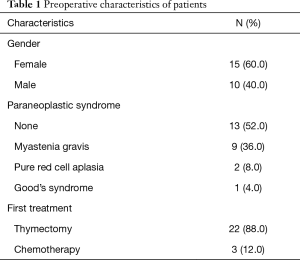
Full table
Peri-operative data
Transesternal approach was used in 72% of cases, and most patients (68%) required the removal of additional structures to achieve negative margins. Detailed data about surgical procedures are shown in Table 2.
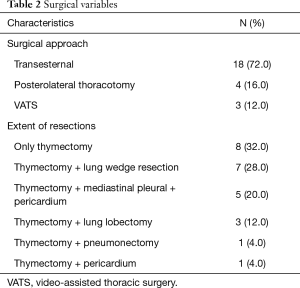
Full table
Median operative time was 197.5 min (88.0–480 min). Median blood loss was 386.6 mL (15–2,000 mL). Postoperative stay on intensive care unit (ICU) was required in 5 cases (20%), with a median stay on ICU of 1.4 days (1–12 days). Median length of stay was 6.4 days (3–18 days). A 90-day morbidity of 24% was reported: 2 cases (8%) with pulmonary embolism, 1 case (4%) of respiratory insufficiency, 1 case with prolonged air-leak (4%), 1 case (4%) with superficial surgical site infection and 1 case (4%) with deep vein thrombosis. A 90-day mortality of 2 cases (8%) was reported (one patient died from massive pulmonary embolism on postoperative day 4; and one patient died of respiratory insufficiency on postoperative day 7).
Based on pathological report, 13 cases (52%) required no adjuvant treatment. Postoperative radiotherapy (PORT) was indicated in 8 cases (32%), all of these cases were Masaoka stage IIB and III. Postoperative chemotherapy was indicated in 7 cases (28%) of IVA thymoma. In 2 patients (8%) with unresectable disease, postoperative concomitant QT/RT was indicated. Of the 12 cases with preoperative paraneoplastic syndrome, a complete remission was achieved in 9 (75%). Detailed pathological results are shown in Table 3.
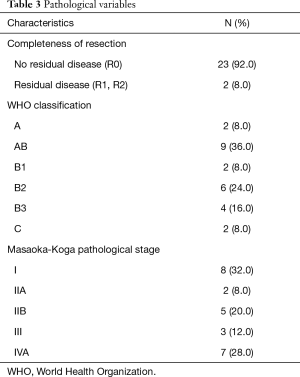
Full table
A complete resection was achieved on 23 cases (92%). On univariate analysis, factors like tumor size, Masaoka-Koga stage or WHO histologic classification were not statistically associated with resectability.
Survival analysis
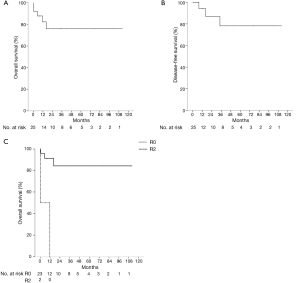
For survival analysis, and due to small sample size patients were grouped according to Masaoka-Koga stage as follows: Group 1, stages I and II; Group 2, stage III and Group 3, stage IV. The 1-, 3- and 5-year OS for Group 1 was 85% for the three time points; for Group 2 was 100% for the three time points; for Group 3 was 57%, 19% and 19% respectively. On a similar way, patients were grouped according to histology as follows: WHO A, WHO AB, WHO B and WHO C. The 1-, 3- and 5-year OS for WHO A was 100% for the three time points; for WHO AB was 88% for the three time points; WHO B was 78% for the three time points; finally, for WHO C was 0%. Only 3 patients had a recurrence (1 case of loco-regional recurrence and 2 cases of pleural metastases). The median follow-up is 34.5 months (1–113 months), median OS and median DFS has not been reached. On univariate analysis the only factor associated with OS was completeness of resection (P<0.0001) (Figure 1). Due to small simple size. Multi-variate analysis was not feasible.
Discussion
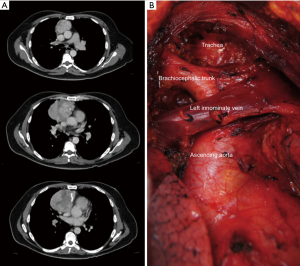
The goal for every thymoma surgery is to obtain clear margins and this requires an aggressive surgical approach for large size tumors. This implies removal of the tumor along with any adjacent tissues that appear to be invaded by thymoma. Completeness of resection is probably the most important prognostic factor as is associated with a better survival and a lower recurrence rate in all series. Although controversy about the need of complete thymus resection has arisen recently (6), the International Thymic Malignancies Interest Group (ITMIG) policies (7) recommends en bloc resection of thymoma including complete thymectomy, plus the surrounding mediastinal fat along with any adjacent structures that may be involved, due to the possibility of invisible invasion of the tumor. Likewise, this has been our institutional policy for all thymectomies (Figure 2).
The Masaoka staging system, further modified by Koga et al., has been the most widely used; however, and due to its limitations the ITMIG and the International Association for the Study of Lung Cancer (IASLC) proposed a new stage system based on the Masaoka-Koga system, but using the TNM components (8). Masaoka-Koga stage has been shown as an independent prognostic factor on most studies. Stages I and II are associated with lower recurrence rates and better OS than stages III and IV (9). A Chinese database including 1,198 patients classified according to IASLC/ITMIG showed a lower recurrence rate for patients with stage I, compared with stages II and IIIa, interestingly, there were no differences between stages II and IIIa. For stages I and II, OS was similar and there were no differences between stages II and IIIa. When compared with stage IIIb, OS was significantly better for stage IIIa tumors. The worst OS was shown by the stage IVb patients, however, there were no significant differences on OS between stages IIIb and IVa or between stage IVa and IVb (10). On a German cohort using the IASLC/ITMIG stage system, the 5-year OS was 73% for all cases, for stage III was 54%, for stage IV was 70%. The 5-year recurrence-free survival was 66% for all cases, for stages I and II was 86%, for stage III 55% and for stage IV 56%. On multivariate analysis, only the stage according to IASLC/ITMIG criteria was associated with OS (P=0.03) (11). Some case series have shown that WHO histology is associated with survival as there is an increasingly higher proportion of higher stage disease moving from A to B3 cases. Nevertheless, the WHO classification has been criticized for poor inter-observer reproducibility and inconsistencies in the routine diagnosis (12). The ITMIG achieved a consensus to refine histological criteria, improve definition and introduce new terms to improve inter-observer reproducibility (13). WHO typification is strongly associated with recurrence rate, in a retrospective review of the ITMIG database, the 5-year recurrence rate was as follows: type A 4%, type AB 2%, type B1 8%, type B2 13% and type B3 14%. In the multivariate analysis, when adjusted to stage, age and resection status, WHO categories were significantly associated with recurrence rate only with stages I and II, but it was not associated with OS (14). Advanced Masaoka-Koga stages such as III and IVa usually invade the mediastinal pleura, pericardium, lungs or great vessels. That tumors have been associated with a higher proportion of incomplete resections, higher recurrence rates and an overall worse prognosis (15).
In a report from the Massachusetts General Hospital, the invasion to other structures was statistically associated on univariate analysis with the risk of recurrence and death. Similarly, tumor size was associated with stage and complete resection. Interestingly, increasing size was associated with recurrences and death. Tumors of 8 cm or larger were more prone to be Masaoka stage III or IV, and more likely to be WHO B1 and higher. On multivariate analysis, Masaoka stage and size were independent factors associated with recurrence (16). In another series, size of thymoma has not been directly associated with survival, but it has been associated with the ability to achieve a complete resection and recurrence (17). In the cohort reported by Safieddine and colleagues on 262 surgically resected cases, patients with thymomas larger than 7.0 cm were associated with an increased risk of recurrence on multivariate analysis (HR 1.2, 95% CI, 1.01–1.43, P=0.04) (18). On the other hand, in a retrospective analysis of the ITMIG database, size was not predictive of R0 resection (19).
Some studies suggest that neo-adjuvant treatment for advanced tumors (specially Masaoka-Koga stage III) could improve rates of complete resection, and consequently improve survival (20,21). Preoperative computed tomography (CT) imaging has been shown to be useful to identify advanced tumors (22). Factors as large size thymoma, lobulated tumor and infiltration to surrounding fat tissue has been associated with stage III Tumors (23,24). Positron emission tomography (PET) has been shown to have a good correlation between SUVmax and WHO histology, with a higher SUVmax on more aggressive tumors (25). Furthermore, relation between SUVmax and Masaoka stage remains controversial (25,26).
As can be noted, despite the advance in imaging techniques, distinguishing a Masaoka-Koga stage II from a stage III requires pathological examination on surgical specimen as the gold standard. Moreover, determining resectability of a thymoma depends not only on imaging appearance, as it should take in account factors related to the patient, and also depends on the experience of the surgeon. Hence, deciding what patient should undergoing neoadjuvant treatment remains complicated. In our cohort, despite the large size of tumors, only 3 patients received neoadjuvant treatment due to uncertainty on complete resection as determined by the thoracic surgery team. Extended resections on thymomas are associated with good perioperative results, in addition to a long DFS and OS (27). In a report from Ried and cols. including 22 patients with Masaoka stage III and IVa who underwent extended resections, intraoperative mortality was 0%, R0 resections were achieved in 86%, recurrence was documented in 22.7% of cases with a median DFS of 30.2 months (28). Huang and colleagues reported on 18 patients with Masaoka stage IVA treated with a multimodal approach including extended resections, all patients received preoperative chemotherapy. There were no intraoperative deaths, morbidity was 39%, an R0 resection was achieved in 67%. The 5- and 10-year OS was 78% and 65% respectively. Median DFS was 95 months (29).
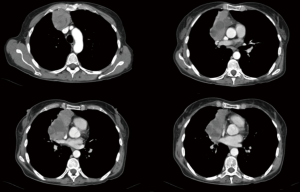
Overall, reported operative mortality for thymectomy is about 2% and morbidity is approximately 20% (2). In our cohort we had a morbidity of 24%, which concur to other series. Conversely, our mortality is above the average, but our population was composed mainly of large and locally advanced tumors, which implies more complex procedures and prolonged operative times. The size ranged from 5 to 14 cm, with a majority of advanced tumors and on 68% of cases the removal of at least one additional structure to complete an en bloc resection was required; regardless of this, a complete resection rate of 92% was achieved, which is very encouraging (Figure 3).
As every retrospective cohort, the present study its limited by its design. Also, the small number of patients and being a single institution report limits the strength of our findings. However, thymomas are a rare disease and randomized trials have not been conducted and most of evidence comes from institutional reports. We are a national reference cancer center in México and usually the most challenging thymic tumors are referred to our clinic. Moreover, to our knowledge, there are very few cohorts that include the number of giant thymomas as in our report.
Conclusions
Size of thymomas should not be considered as a contraindication for surgical treatment. Large size thymomas are associated with advanced Masaoka stage requiring extended resections, but according to our data surgery is feasible, even in advanced cases and provides the best chance for cure. En bloc removal must be the goal in every thymoma surgery as it guarantees a high proportion of R0 resections. Complete resection remains as one of the most important prognostic factor in thymomas and is associated with prolonged DFS and OS.
Acknowledgements
None.
Footnote
Conflicts of Interest: Parts of this data were presented at the IASLC 18th World Conference on Lung Cancer on October 2017.
Ethical Statement: The study was approved by the Institutional Ethics Committee (No. INCAN/CI/837/17), which waived the requirement of an individual patient consent because only non-sensible data were used for the analysis.
References
- Falkson CB, Bezjak A, Darling G, et al. The Management of Thymoma: A systematic Review and Practice Guideline. J Thorac Oncol 2009;4:911-9. [Crossref] [PubMed]
- Detterbeck FC, Parsons AM. Management of stage I and II thymoma. Thorac Surg Clin 2011;21:59-67. vi-vii. [Crossref] [PubMed]
- Engels EA. Epidemiology of Thymoma and Associated Malignancies. J Thorac Oncol 2010;5:S260-5. [Crossref] [PubMed]
- Detterbeck F, Youssef S, Ruffini E, et al. A Review of Prognostic Factors in Thymic Malignancies. J Thorac Oncol 2011;6:S1698-704. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Staging of thymic epithelial neoplasms: Thymoma and thymic carcinoma. Pathol Res Pract 2015;211:2-11. [Crossref] [PubMed]
- Bae MK, Lee SK, Kim HY, et al. Recurrence after thymoma resection according to th extent od the resection. J Cardiothorac Surg 2014;9:51. [Crossref] [PubMed]
- Detterbeck FC, Moran C, Huang J, et al. Which way is up? Policies and procedures for surgeons and pathologist regarding resection specimens of thymic malignancy. J Thorac Oncol 2011;6:S1730-8. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: Proposal for an Evidence-Based Stage Classification System for the Forthcoming (8th) Edition of the TNM Classification of Malignant Tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Filosso PL, Ruffini E, Lausi PO, et al. Historical perspectives: The evolution of the thymic epithelial tumors staging system. Lung Cancer 2014;83:126-32. [Crossref] [PubMed]
- Liang G, Gu Z, Li Y, et al. Comparison of the Masaoka-Koga staging and the International Association for the Study of Lung Cancer/the International Thymic Malignancies Interest Group proposal for the TNM staging systems based on the Chinese Alliance for Research in Thymomas retrospective database. J Thorac Dis 2016;8:727-37. [Crossref] [PubMed]
- Ried M, Eicher MM, Neu R, et al. Evaluation of the new TNM-staging system for thymic malignancies: impact on indication and survival. World J Surg Oncol 2017;15:214. [Crossref] [PubMed]
- Wu J, Fang W, Chen G. The enlightenments from ITMIG Consensus on WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Dis 2016;8:738-43. [Crossref] [PubMed]
- Marx A, Ströbel P, Badve SS, et al. ITMIG Consensus Statement on the Use of the WHO histological Classification of Thymoma and Thymic Cracinoma: Refined Definitions, Histological Criteria, and Reporting. J Thorac Oncol 2014;9:596-611. [Crossref] [PubMed]
- Weis CA, Yao X, Deng Y, et al. The Impact of Thymoma Histotype on Prognosis in a Worldwide database. J Thorac Oncol 2015;10:367-72. [Crossref] [PubMed]
- Wright CD. Extended Resections for Thymic Malignancies. J Thorac Oncol 2010;5:S344-7. [Crossref] [PubMed]
- Wright CD, Wain JC, Wong DR. Predictors of recurrence in thymic tumors: importance of invasión, WHO histology and size. J Thorac Cardiovasc Surg 2005;130:1413-21. [Crossref] [PubMed]
- Ruffini E, Venuta F. Management of thymic tumors: a European perspective. J Thorac Dis 2014;6:S228-37. [PubMed]
- Safieddine N, Liu G, Cuningham K, et al. Prognostic Factors for Cure, Recurrence and Long-Term Survival after Surgical Resection of Thymoma. J Thorac Oncol 2014;9:1018-22. [Crossref] [PubMed]
- Burt BM, Yao X, Shrager J, et al. Determinants of Complete Resection of Thymoma by Minimally Invasive and Open Thymectomy: Analysis of an International Registry. J Thorac Oncol 2017;12:129-36. [Crossref] [PubMed]
- Carillo C, Diso D, Mantovani S, et al. Multimodality Treatment of Stage II Thymic Tumours. J Thorac Dis 2017;9:2369-74. [Crossref] [PubMed]
- Venuta F, Rendina EA, Longo F, et al. Ann Thorac Surg 2003;76:1866-72. [Crossref] [PubMed]
- Hayes SA, Huang J, Plodkowski AJ, et al. Preoperative Computed Tomography Findings Predict Surgical Resectability of Thymoma. J Thorac Oncol 2014;9:1023-30. [Crossref] [PubMed]
- Marom EM, Milito MA, Moran CA, et al. Computed Tomography Findings Predicting Invasiveness of Thymoma. J Thorac Oncol 2011;6:1274-81. [Crossref] [PubMed]
- Qu YJ, Liu G, Shi H, et al. Preoperative CT Findings of Thymoma are Correlated with Postoperative Masaoka Clinical Stage. Acad Radiol 2013;20:66-72. [Crossref] [PubMed]
- Park SY, Cho A, Bae MK, et al. Value of 18-FDG PET/CT for Predicting the World Health Organization Malignant Grade of Thymic Epithelial Tumors: Focused in Volume-Dependent Parameters. Clin Nucl Med 2016;41:15-20. [Crossref] [PubMed]
- Korst RJ, Fernando S, Catlin AC, et al. Positron Emission Tomography in Thymic Tumors: Analysis Using a Prospective Research Database. Ann Thorac Surg 2017;104:1815-20. [Crossref] [PubMed]
- Rea F, Marulli G, Girardi R, et al. Long-term survival and prognostic factors in thymic epitelial tumours. Eur J Cardiothorac Surg 2004;26:412-8. [Crossref] [PubMed]
- Ried M, Potzger T, Sziklavari Z, et al. Extended Surgical Resections od Advanced thymoma Masaoka syages III and IVa Facilitate Outcome. Thorac Cardiovasc Surg 2014;62:161-8. [PubMed]
- Huang J, Rizk NP, Travis WD, et al. Feasibility of Multi-Modality Therapy Including Extended Resections in Stage IVA Thymoma. J Thorac Cardiovasc Surg 2007;134:1477-83; discussion 1483-4. [Crossref] [PubMed]


