Paraganglioma presenting as acute myocardial infarction
Introduction
Paragangliomas are neuroendocrine tumors that originate in the parasympathetic ganglia. These cells normally act as special chemoreceptors located along blood vessels, particularly near the carotid bodies and aortic bodies. Paragangliomas include asymptomatic chromaffin-negative tumors and chromaffin-positive tumors that secrete high amounts of catecholamines, mostly norepinephrine, plus epinephrine, causing corresponding symptoms. Pheochromocytomas and paragangliomas (PPGLs) are estimated to occur in about 2–8 of 1 million persons per year and about 0.1% of hypertensive patients harbor a PPGL. And in approximately twenty percent of these tumors are paragangliomas (1).
Case presentation
A 48-year-old woman with eumenorrhea had a history of hypertension for 5 years. Her blood pressure (BP) was usually within the range from 120/70 to 200/100 mmHg, with occasionally episodes of palpitations, dizziness and headache. She took Amlodipine for her BP but she did not routinely monitor her BP. She denied chest pain, smoking, alcohol abuse, diabetes, dyslipidemia or cerebral infarction.
The patient had sudden persistent severe upper abdominal pain for 3 days and then went to the local hospital. Abdominal CT demonstrated a retroperitoneal occupying lesion. She was given meperidine which improved symptoms slightly without electrocardiogram or troponin examination. The patient was transferred to our hospital for surgical treatment. In the emergency department, sudden hemodynamic collapse (BP and pulse are unavailable) was noted, and the patient lost consciousness at the same time. After immediate cardiopulmonary resuscitation for 1 minute, the patient had autonomous cardiac rhythm and her BP improved to 90/50 mmHg. Her electrocardiogram (ECG) showed 0.5–1.5 mm ST-segment elevation in lead II, III, and aVF and lowering and inversion of T-wave in lead I, V5, and V6 (Figure 1). Biochemical analysis demonstrated elevated levels of white cells counts, CK myocardial isoenzyme (CK-MB, 137 U/L; reference range, 0–23 U/L) and Cardiac troponin T (cTnT, 0.972 ng/mL; reference range, <0.03 ng/mL) with low serum potassium (3.3 mmol/L; reference range, 4.5–5.3 mmol/L). Echocardiography showed decreased contractility in the left ventricle, slightly hypertrophic left ventricular wall and left ventricular ejection fraction (LVEF) of 58%. Acute coronary syndrome was considered. Treatment with aspirin, ticagrelor, low molecular weight heparin, atorvastatin, isosorbide mononitrate, benazepril hydrochloride, and metoprolol was initiated and the patient was admitted to the cardiology ward.
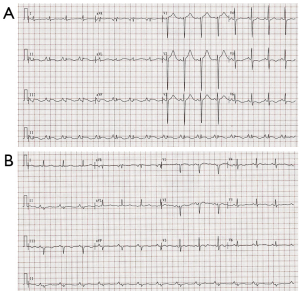
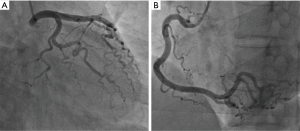
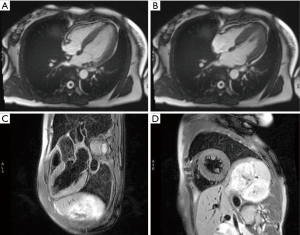
After admission, coronary angiography showed normal coronary artery (Figure 2). Cardiac troponin T level decreased gradually to normal. ECG demonstrated reduction of ST-segment to baseline in leads II, III, and aVF, and Q-wave in leads III and avF (Figure 1B). Echocardiography showed slightly decreased contractility in the left ventricle, an improvement from before, and LVEF of 63%. Enhanced MRI did not show abnormalities, with LVEF of 66% (Figure 3). The patient had paroxysmal BP elevation up to 210/160 mmHg twice during hospitalization with dizziness and headache. 20 minutes after the oral administration of captopril (12.5 mg), the BP decreased to 130/80 mmHg and symptoms were relieved. Biochemical analysis demonstrated elevation levels of plasma metanephrine (957.93 pg/mL, reference range, <96.6 pg/mL) and methoxyepinephrine (1,069.82 pg/mL, reference range, <163 pg/mL). Abdominal enhanced MRI was arranged and revealed an obvious enhanced irregular soft tissue mass about 3.5×5.5 cm2 at the right side of aorta abdominals below the coeliac trunk with non-enhancing patchy hypodense area inside (Figure 4). Paraganglioma was considered.

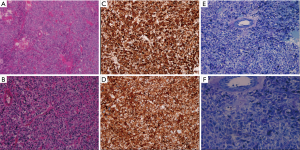
The results above suggested a paraganglioma. The patient subsequently underwent excision of the retroperitoneal tumor under general anesthesia followed by oral administration of benazepril, doxazosin, and metoprolol for 1 month. During the operation, a palpable tumor rich in blood vessels was found in the posteroinferior margins of the pancreas, around the renal hilum, between the inferior vena cava and abdominal aorta, next to the abdominal aorta. The longest diameter was 5 cm. BP fluctuated widely during the operation, up to 200/120 mmHg. Paraganglioma was confirmed by postoperative pathologic findings. There was capsular invasion in focal areas and suspicious tumor thrombi in vessels (Figure 5). The patient did not have fluctuation of BP, abdominal pain and cardiac arrest postoperatively. Follow-up echocardiography did not show any abnormalities. Levels of plasma catecholamine also returned to normal.
Discussion
Ninety percent of paragangliomas are chromaffin-positive and catecholamine-secreting tumors. The most common symptoms are paroxysmal hypertension and symptoms of paroxysmal adrenergic stimulation, like headache, pallor, diaphoresis, tachycardia, chest discomfort, nausea, vomiting, and blurred vision. In a few cases, patients may present with hypotension, shock, or alternation of hypertension and hypotension, arrhythmia, and cardiomyopathy (2,3). However, the absence of these symptoms can present a considerable diagnostic challenge. Therefore, it is easy misdiagnose or miss the diagnosis until serious complications occur. Our case presented as acute myocardial infarction and cardiac arrest. Proposed mechanisms include: (I) acute catecholamine secretion can activate α receptor in vessels, leading to severe coronary vasoconstriction and myocardial ischemia and necrosis. Sudden cardiac arrest caused by malignant arrhythmias can also be observed. Blood supply is recovered after relieving coronary spasms; (II) sustained or acute elevation of plasma catecholamine can lead to myocardial injury, resulting in myocardial stunning or sudden cardiac arrest caused by malignant arrhythmias. It has been reported that eleven percent of paraganglioma patients presented with acute catecholamine cardiomyopathy. However the cardiomyopathy is usually reversible with similar mechanisms like those seen in Takotsubo cardiomyopathy (4,5). But paraganglioma patients are more vulnerable to serious complications such as shock and heart failure (6) Adrenergic α-antagonists, selective adrenergic β1-antagonists, and ACEIs were used to counter overactivity of the sympathetic nervous system. Thus the BP can be controlled and myocardial damage and malignant arrhythmia can be prevented. However, the essential treatment is early surgical excision.
In our case, the patient presented as acute myocardial infarction and cardiac arrest with normal coronary artery on angiography. A history of paroxysmal hypertension raised the suspicion of a paraganglioma. Elevated levels of plasma catecholamine and imaging studies supported the diagnosis. The diagnosis is confirmed by operation and pathology. We suggest a careful enquiring of patients’ clinical history when they present as acute coronary syndrome with normal coronary artery on angiography. Especially when patients have acute abdominal pain, abdominal mass, paroxysmal hypertension and other related medical history, paraganglioma should be included in the differential diagnosis of acute coronary syndrome.
Acknowledgements
Funding: This case report was supported in part by the grant 81700441 (J Hu) from The National Natural Science Foundation of China, by the grant 81670460 (Y Yan) from the National Nature Science Foundation, Beijing, China.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Pacak K, Tella SH. Pheochromocytoma and Paraganglioma. In: De Groot LJ, Chrousos G, Dungan K, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000.
- Lenders JW, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. Lancet 2005;366:665-75. [Crossref] [PubMed]
- Liao WB, Liu CF, Chiang CW, et al. Cardiovascular manifestations of paraganglioma. Am J Emerg Med 2000;18:622-5. [Crossref] [PubMed]
- Giavarini A, Chedid A, Bobrie G, et al. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart 2013;99:1438-44. [Crossref] [PubMed]
- Lyon AR, Rees PS, Prasad S, et al. Stress (Takotsubo) cardiomyopathy--a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med 2008;5:22-9. [Crossref] [PubMed]
- Agarwal V, Kant G, Hans N, et al. Takotsubo-like cardiomyopathy in paraganglioma. Int J Cardiol 2011;153:241-8. [PubMed]


