Clinical assessment of airway stent placement in patients with malignant airway lesions
Introduction
Central airway obstruction (CAO) or tracheoesophageal fistulas are life-threatening conditions of progressive stage lung cancer (LC) patients or esophageal cancer (EC) patients after esophagectomy. CAO is defined as occlusion of >50% of the trachea, main bronchi, bronchus intermedius, or a lobar bronchus. Malignant CAO can cause clinical symptoms including dyspnea, stridor and obstructive pneumonia, which account for 20–30% of patients with primary LC (1-3).
Airway stenting is the optimal palliation therapy for advanced cancer patients with extrinsic compression, endoluminal tumors or tracheoesophageal fistulas. By maintaining airway patency in patients with malignant airway stenosis and establishing airway integrity in patients who suffer from tracheoesophageal fistulas, stent placement can improve symptoms rapidly. However, stents-related complications also occur, including mucous plugging, tumor restenosis, stent migration, granulation tissues formation, fistula enlargement, stent fracture, perforation and hemorrhage. These short- or long-term complications can occur with both silicone stents and self-expandable metallic stents (SEMSs) (4,5).
Therapeutic bronchoscopy includes stent placement; to be more specific, it also contains the technique for tumor debulking, which includes mechanical debulking, thermocoagulation, argon-plasma coagulation, laser therapy, cryotherapy, balloons dilation and microdebriders (4). It has been reported that the 30-day mortality after placement of stents was 14.8%, and one of the risk factors for increased 30-day mortality was stent deployment. Thus, the balance between intervention and expected complications is very important for the clinical application of the stent (6). Some bronchoscopists have reported their single or multi-center experiences with the effectivity of airway stenting and accompanied procedural complications; however, few studies have analyzed the risk factors for each stent-related complication or compared the incidence of complications between different cancers (7-15).
The present study aimed to assess the application of airway stents, including the clinical effects, and to explore the high-risk factors for specific complications. We described our monocentric experience to determine the safety and feasibility for the deployment of SEMSs or silicone stents in the management of patients with malignant airway obstruction or fistulas.
Methods
Patients
This study includes a retrospective cohort of LC patients and EC patients with malignant CAO or tracheoesophageal fistulas who presented for placement of an airway stent in parallel between January 2014 and July 2017 at our center. Ethics review approval from the First Affiliated Hospital, Zhejiang University School of medicine was obtained for this study (ethics approval number: 2017-668).
Symptomatic patients with malignant CAO or tracheoesophageal fistula of the trachea, carina, or lobar bronchia had a flexible bronchoscopy and computed tomography (CT) scan of the chest. A full medical history, physical examination, CT imaging, type of stents, type of bronchoscope, treatment modalities, operation-related complications and outcomes were assessed by electronic medical records. The Charlson comorbidity index (CCI) was calculated to evaluate the physical condition in each patient. The severity of stenosis or fistulas and the diameter and length of the trachea were measured by 3-dimensional (3D) airway reconstruction. In all the patients, dyspnea or dysphagia grading and Karnofsky Performance Status (KPS) were precisely assessed before and after the placement of stents. The first follow-up bronchoscopy was performed 24 hours after stent placement.
During the follow-up period, esophageal and respiratory symptoms as well as dyspnea or dysphagia grading were monitored. All the possible stent-related complications were confirmed with bronchoscopic and 3D airway reconstruction. Flexible bronchoscopy was used to reassess the patency and stent position. The follow-up data were obtained from the outpatient clinic chart reviews systems or by telephone calls to patients. The last follow-up occurred in August 2017.
Stent placement
The procedures for the placement of the SEMSs were as follows. First, bronchoscopists checked the position of stenosis or fistula by a bronchoscope. Next, the guide-wires were inserted into the diseased trachea or the right and left bronchi. Under bronchoscopic guidance, the delivery system was advanced over the guide-wires into the position of the carina. By retracting the introducer sheath rapidly, the stents were then released. After confirmation that the stents were deployed at the right level, bronchoscopists completely withdrew the introducer sheath and guide-wires.
General anesthesia was required for rigid bronchoscopy (Karl-Storz, Tuttlingen, Germany), while the procedure using the flexible bronchoscopy (BF 1T260, Olympus, Tokyo, Japan) was performed under local anesthesia by sprinkling 2–3 mL of 2% lidocaine via the catheter.
The self-expanding covered metallic stent had a tracheal limb measuring 10 to 22 mm in diameter and 20 to 100 mm in length. For the Y stent, the diameter of the left or right main bronchi varied from 10 to 18 mm, and the length varied from 10 to 40 mm. The size of the stents was customized to fit different patients’ airways. A silicone stent was seldom used for therapeutic bronchoscopy in our center. During the placement, if needed, we would inflate an expansion balloon three or four times for 20 seconds, and argon plasma coagulation (40-Watt, blended mode-continuous flow) was used for cutting the neoplasm in the airway.
Statistical analysis
Binary logistic regression models were adopted to determine the association between stent-related complications and known covariates including gender, age, presence of malignant disease, pathology, history of smoking or drinking, CCI, pre-stent therapy, stent location, airway situation, deployment duration and stent length. Continuous variables were compared by Student’s t-test and summarized by means and ranges. The categorical data were compared using a Fisher’s exact text and summarized with frequencies. A two-sided significance level of 0.05 was used for all the statistical tests, unless specifically clarified. All the statistical analyses were performed using SPSS software 24.0 (IBM statistics, SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Between January 2014 and July 2017, a total of 56 patients with malignant airway stenosis or fistula underwent stent placement. Twenty-five patients (12 in LC group, 13 in EC group) received a total of 33 Y-shaped stents. Only 3 patients received Y-shaped silicone stents. For metallic tube stents, 31 patients (17 in LC group, 14 in EC group) received a total of 33 stents. The baseline characteristics of these patients and the univariate analysis of the risk factors for the overall complications are shown in Table 1.
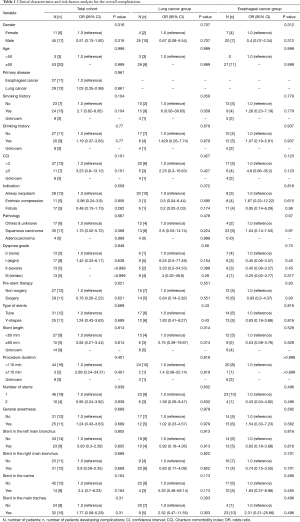
Full table
Clinical outcome
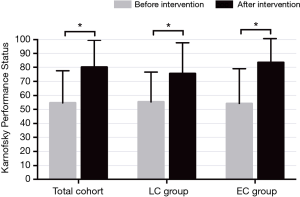
The stents were all successfully deployed, and there were no acute complications with any stent placement. More than half of the patients (66%) had remarkably downgraded dyspnea levels after the intervention therapy. Sixteen (28%) patients whose dyspnea grade was equal or less than grade I maintained their former grades. Additionally, three patients underwent mechanical ventilation after placement so that dyspnea grade was not possible to assess. The 24-hour post-stent placement mean KPS significantly improved (79.05±20.71 vs. 56.67±23.52, P<0.001). By the subgroup analysis, the KPS improved in both the LC and EC groups (LC group: 55.45±21.15 vs. 75.45±22.07; P=0.001, and EC group: 54.29±24.72 vs. 83.57±16.92; P<0.001) (Figure 1). No patients died in the hospital. However, 10 patients (17.9%) died within 30 days.
Risk factors for specific complications
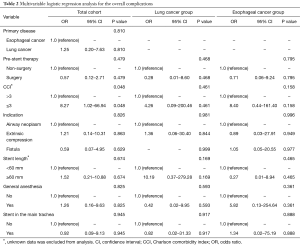
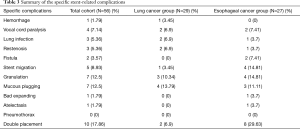
The multivariable analysis showed that patients with low CCI (<3) (P=0.048) were associated with an increased risk for overall complications (Table 2). The summary of specific stent-related complications is listed in Table 3. Fourteen percent of patients had more than two complications. All the complications occurred in the patients with metallic stents. The incidence of specific complications was similar in both groups. The median follow-up duration was 545 days.
Full table
Full table
We thoroughly analyzed all the potential risk factors in the total cohort, including the LC group and the EC group (Table S1), and identified the high-risk factors (Table 4). Among all the patients, pre-stent non-surgical therapy (P=0.048) and general anesthesia (P=0.038) were associated with an increased risk of granulation. Low CCI (<3) (P=0.008) and a long procedure duration (>110 min) (P=0.005) were associated with increased risk for restenosis. Stents only in the main trachea (P=0.049) led to stent migration. In addition, pre-stent surgical therapy (P=0.05) and extrinsic compression (P=0.058) had a marginal effect on migration. A tubular stent in the left/right bronchus (P=0.023) had a significant impact on atelectasis. Stenting the carina or the upper airways was associated with mucous plugging (P=0.041). Double placement was more common in EC patients (P=0.04), where pre-stent surgical therapy (P=0.012) and low CCI (<3) (P=0.028) became two high-risk factors for double placement. In the LC group, small cell lung cancer (P=0.035) and the stent length (>60 mm) (P=0.003) increased the risk of atelectasis and mucous plugging, respectively. In the EC group, a long procedure duration (>110 min) (P=0.037) and the stent length (>60 mm) (P=0.019) were associated with a higher incidence of lung infection and granulation, respectively.
Full table
Full table
Discussion
In this study, we revealed the efficacy and feasibility of placing airway stents for the management of malignant airway lesions, including airway neoplasm (Figure 2), tracheoesophageal fistula and extrinsic compression (Figure 3). The improvement of dyspnea grades and KPS suggested the immediate relief of symptoms. Almost all the patients received metallic stents because the advantages of metallic stents for treating malignant airway lesions were obvious, including higher long-time stent patency rates and lower migration rates (7,8). However, high complication rates have restricted the use of metallic stents. The Food and Drug Administration (FDA) advised the use of SEMSs only in cases in which surgery or placement of silicone stents were ineligible (9). In the current study, no significant factor was identified to increase the risk of overall complications. Nonetheless, the results might be affected by many factors, such as the different comorbidities, various baseline characteristics and patient heterogeneity. Then, we focused on each specific stent-related complication and made assessments systematically on this issue.
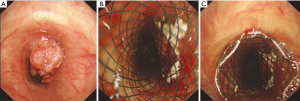

We found that pre-stent non-surgical therapy and general anesthesia were associated with higher granulation rates. Ingrowth of granulation tissue is a well-recognized complication for metallic stent placement with an occurrence rate of 20% (5,10-12). The higher incidence rates of granulation tissue were related to the underlying malignancies. Prior studies have shown that trauma and bacterial contamination can cause granulation (13). Additionally, general anesthesia was required for rigid bronchoscope, which may cause trauma, and the longer operation duration compared with conscious sedation increased the potential infection rates. A prior study suggested that conscious sedation was associated with increased complication rates (6). We also observed that the length of stents (>60 mm) was a risk factor for granulation in the EC group. Longer stents mean a larger contact area on the inner surface of the trachea, with much more mucosal inflammation and granulation formation.
Several studies have reported the incidence of stent restenosis, ranging from 5% to 19.4% (13-16). The main causes of restenosis are the overgrowth of endoluminal tumors or granulation tissue, followed by fibrosis. Tumor or granulation tissue grows at the covered stent ends or through the uncovered stent wires. Our results suggest that a CCI <3 and a procedure duration >110 min are associated with an increased risk of restenosis. It is worth noting that a higher CCI was associated with a higher probability of deterioration after bronchoscopic therapy. The short life expectancy of patients with a high CCI may be inadequate for the development of long-term complications, such as stent restenosis. In our center, all the cases of restenosis were caused by fibrosis, and all of these patients suffered from airway neoplasm and received debulking therapies, including electrocautery, argon plasma coagulation or cryotherapy. All the debulking therapies were time-consuming and traumatic.
In comparison with silicone stents, SEMSs had a lower rate of migration due to their better resistance, pliability and lower contraction (13,17-20), especially for the Y-shaped stents (14,16,21,22). However, migration in the metallic stents still occurred because of stent under-sizing, extrinsic compression, severer cough, and bad expanding or shrinkage of the tumor following chemotherapy and/or radiotherapy (15). These results are consistent with our analysis that the patients with extrinsic compression and stents only in the main trachea had a higher incidence of migration.
Mucous plugging, as a common stent-related complication, has a high occurrence rate (5,11). In our study, 12.5% of patients developed mucous plugging, and stenting in the carina or the upper airways was a risk factor for this complication. Covered stents played an important role in the occurrence of mucous plugging. The main cause of mucous retention is dysfunction of the cilia when a covered metallic stent is placed. A longer length of a covered stent results in an increase in dysfunctional cilia, which could explain why long stents (>60 mm) were associated with a greater risk of mucous plugging in the LC group. The irritation from the stent or operation induced severe cough, which then increased mucous production. Therefore, nebulization should be routinely used for these patients, and phlegm suctioning should be performed using fiberoptic bronchoscopy when necessary.
Prior studies have suggested that infections are more common in patients undergoing airway interventions, and these infections were associated with an increased 30-day mortality rate (6,13,23). Additionally, infections were also a risk factor for granulation formation (13). Whereas the infection rates after stent placement are rarely reported in recent studies, the reported infection rates range widely from 5.7% to 40% (1,5,16). Only 3 (5.4%) cases of lung infection were detected in this study, and a long procedure duration (>110 min) was a risk factor for a lung infection in the EC group.
A large improvement of symptoms and an immediate palliation of CAO or airway fistulas have been observed in many studies (24-30). However, complications after stent placement are inevitable due to the limitations of materials and bronchoscopic technology. Nevertheless, the specific complications can be managed with many feasible treatments. The rapid treatment of complications will result in greater benefits for patients. Thus, bronchoscopists should pay particular attention to high-risk factors for stent-related complications and take prompt and proactive actions to manage complications to eliminate the suffering of patients.
Limitations of this study include the relatively small sample size and the monocentric design. This study was not a blinded, randomized, controlled or prospective study. We did not find any evidence that stent placement impacted the survival.
In conclusion, this is the first study of airway stents to systematically analyze and investigate the risk factors for each stent-related complication. Our results suggested that granulation rates were higher in patients who underwent general anesthesia and those who did not received surgery before stenting. Restenosis rates were higher in patients who had a low CCI or a long duration of the intervention. The stent location was strongly associated with stent migration and mucus impaction. Additionally, our findings also revealed that the length of the stent was directly associated with mucous plugging and granulation tissue formation. We believe that our findings will assist clinicians and bronchoscopists in quickly identifying complications and devising early interventions to manage stent-related complications in patients with malignant airway lesions.
Acknowledgements
Funding: This work was supported by The National Key R&D Program of China, with the grant number 2017YFC0113500, The Key Project of Zhejiang Province Science and Technology Plan, China with grant number 2014C03032 and The Key Project of Zhejiang Province Traditional Chinese Medicine Science and Technology Plan, China with grant number 2015ZZ007. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethics review approval from the First Affiliated Hospital, Zhejiang University School of medicine was obtained for this study (ethics approval number: 2017-668).
References
- Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278-97. [Crossref] [PubMed]
- Oviatt PL, Stather DR, Michaud G, et al. Exercise capacity, lung function, and quality of life after interventional bronchoscopy. J Thorac Oncol 2011;6:38-42. [Crossref] [PubMed]
- Semaan R, Yarmus L. Rigid bronchoscopy and silicone stents in the management of central airway obstruction. J Thorac Dis 2015;7:S352-62. [PubMed]
- Guibert N, Mhanna L, Droneau S, et al. Techniques of endoscopic airway tumor treatment. J Thorac Dis 2016;8:3343-60. [Crossref] [PubMed]
- Murgu SD, Egressy K, Laxmanan B, et al. Central airway obstruction: Benign strictures, tracheobronchomalacia, and malignancy-related obstruction. Chest 2016;150:426-41. [Crossref] [PubMed]
- Ost DE, Ernst A, Grosu HB, et al. Complications Following Therapeutic Bronchoscopy for Malignant Central Airway Obstruction: Results of the AQuIRE Registry. Chest 2015;148:450-71. [Crossref] [PubMed]
- Ayub A, Al-Ayoubi AM, Bhora FY. Stents for airway strictures: Selection and results. J Thorac Dis 2017;9:S116-21. [Crossref] [PubMed]
- Yuan TW, Liu HQ, Wang SB, et al. Comparison of plastic stents with self-expandable metal stents in palliative treatment of malignant biliary obstruction: A meta-analysis. Eur Rev Med Pharmacol Sci 2017;21:2847-57. [PubMed]
- Lund ME, Force S. Airway stenting for patients with benign airway disease and the food and drug administration advisory. Chest 2007;132:1107-8. [Crossref] [PubMed]
- Chung FT, Chen HC, Chou CL, et al. An outcome analysis of self-expandable metallic stents in central airway obstruction: a cohort study. J Cardiothorac Surg 2011;6:46. [Crossref] [PubMed]
- Herth FJ, Eberhardt R. Airway stent: What is new and what should be discarded. Curr Opin Pulm Med 2016;22:252-6. [Crossref] [PubMed]
- Nam HS, Um SW, Koh WJ, et al. Clinical application of the Natural Y stent in the management of benign carinal stenosis. Ann Thorac Surg 2009;88:432-9. [Crossref] [PubMed]
- Ost DE, Shah AM, Lei X, et al. Respiratory Infections Increase the Risk of Granulation Tissue Formation Following Airway Stenting in Patients With Malignant Airway Obstruction. Chest 2012;141:1473-81. [Crossref] [PubMed]
- Breitenbücher A, Chhajed PN, Brutsche MH, et al. Long-term follow-up and survival after ultraflex stent insertion in the management of complex malignant airway stenoses. Respiration 2008;75:443-9. [Crossref] [PubMed]
- Chhajed PN, Somandin S, Baty F, et al. Therapeutic bronchoscopy for malignant airway stenoses: Choice of modality and survival. J Cancer Res Ther 2010;6:204-09. [Crossref] [PubMed]
- Serrano C, Laborda A, Lozano JM, et al. Metallic stents for tracheobronchial pathology treatment. Cardiovasc Intervent Radiol 2013;36:1614-23. [Crossref] [PubMed]
- Dalar L, Ozdemir C, Abul Y, et al. Therapeutic bronchoscopic interventions for malignant airway obstruction: A retrospective study from experience on 547 patients. Medicine (Baltimore) 2016;95. [Crossref] [PubMed]
- Ma J, Han X, Wu G, et al. Outcomes of temporary partially covered stent placement for benign tracheobronchial stenosis. Cardiovasc Intervent Radiol 2016;39:1144-51. [Crossref] [PubMed]
- Matsumoto K, Yamasaki N, Tsuchiya T, et al. Double stenting with silicone and metallic stents for malignant airway stenosis. Surg Today 2017;47:1027-35. [Crossref] [PubMed]
- Verma A, Phua CK, Wu QM, et al. Our Clinical Experience of Self-Expanding Metal Stent for Malignant Central Airway Obstruction. J Clin Med Res 2017;9:58-63. [Crossref] [PubMed]
- Li TF, Duan XH, Han XW, et al. Application of combined-type Y-shaped covered metallic stents for the treatment of gastrotracheal fistulas and gastrobronchial fistulas. J Thorac Cardiovasc Surg 2016;152:557-63. [Crossref] [PubMed]
- Yang RM, Han XW, Wu G, et al. Implantation of a self-expandable metallic inverted y-stent to treat tracheobronchial stenosis in the carinal region: Initial clinical experience. Clinical Radiology 2007;62:1223-8. [Crossref] [PubMed]
- Grosu HB, Eapen GA, Morice RC, et al. Stents are associated with increased risk of respiratory infections in patients undergoing airway interventions for malignant airways disease. Chest 2013;144:441-9. [Crossref] [PubMed]
- Agustsson T, Nilsson M, Henriksson G, et al. Treatment of postoperative esophagorespiratory fistulas with dual self-expanding metal stents. World J Surg 2009;33:1224-8. [Crossref] [PubMed]
- Fortin M, Lacasse Y, Elharrar X, et al. Safety and Efficacy of a Fully Covered Self-Expandable Metallic Stent in Benign Airway Stenosis. Respiration 2017;93:430-5. [Crossref] [PubMed]
- Husain SA, Finch D, Ahmed M, et al. Long-term follow-up of ultraflex metallic stents in benign and malignant central airway obstruction. Ann Thorac Surg 2007;83:1251-6. [Crossref] [PubMed]
- Jeong BH, Um SW, Suh GY, et al. Results of interventional bronchoscopy in the management of postoperative tracheobronchial stenosis. J Thorac Cardiovasc Surg 2012;144:217-22. [Crossref] [PubMed]
- Madan K, Dhooria S, Sehgal IS, et al. A Multicenter Experience With the Placement of Self-Expanding Metallic Tracheobronchial Y Stents. J Bronchology Interv Pulmonol 2016;23:29-38. [Crossref] [PubMed]
- Mahmood K, Wahidi MM, Thomas S, et al. Therapeutic bronchoscopy improves spirometry, quality of life, and survival in central airway obstruction. Respiration 2015;89:404-13. [Crossref] [PubMed]
- Mitsuoka M, Sakuragi T, Itoh T. Clinical benefits and complications of dumon stent insertion for the treatment of severe central airway stenosis or airway fistula. Gen Thorac Cardiovasc Surg 2007;55:275-80. [Crossref] [PubMed]


