A missed case of pulmonary histiocytosis in a young lung donor
Introduction
Lung transplantation has definitely been recognized as the treatment of choice for different end-stage lung diseases (1).
The availability of lung donors is the critical point in the transplant field because the lung is a very susceptible organ, with reported acceptance rates varying from 15% to 35% among centers (2).
Indeed, several lesions causing brain death can lead to significant pathologic pulmonary changes such as lung contusion, infection, aspiration or neurogenic pulmonary edema.
During graft retrieval, a correct organ evaluation based on clinical-functional parameters and on surgical experience is mandatory for a good postoperative outcome.
In recent years, the introduction of ex vivo lung perfusion (EVLP) has significantly changed the timing of donor evaluation, postponing the final decision for organ selection to the “ex vivo time” (3,4). However, a detailed collection of anamnestic data from relatives and overall a careful lung evaluation, are still today important parts in the decision-making process to assess the donor suitability.
Case presentation
We report the case of a 19-year-old female multi-organ donor who was admitted to the intensive care unit (ICU) due to a spontaneous cerebral hemorrhage. Clinical/anamnestic data were collected from her parents and did not indicate any pathological abnormalities; in particular, there was no history of cigarette smoking. On admission, the patient underwent chest X-ray and a total body computed tomography (CT) scan that did not show any remarkable lung alterations even though they were evaluated by the local radiologist and by the retrieval surgeon. After the declaration of brain death (12 days after admission to the ICU) and the parents’ consent, the patient was considered a standard multi-organ donor. Donor Oto score was 0 with a paO2/FiO2 of 555 mmHg; bronchoscopy showed no alterations with only positivity for Staphylococcus Aureus on bronchial aspirate.
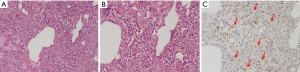
Ten hours later the donor went into the operating room for retrieval during which a loss of lung parenchyma elasticity was observed, associated with a low degree of pO2/FiO2 ratio (250 mmHg). Different strategies for parenchymal lung recruitment (aspiration, diuretics, manual ventilation) were performed but they were all ineffective (despite the use of pulmonary vein sampling); a careful tactile evaluation was performed but the consistency of the graft was definitively not convincing. At that time, our transplant center did not have an EVLP system available but we are quite sure that even we had had the opportunity to recondition the lungs with EVLP we would not have accepted them for the implantation because of the unconvincing lung parenchyma. The lungs were then rejected and a small parenchymal resection, corresponding to an area of increased consistency on palpation, was sent for pathological examination. Histological and immunohistochemical analyses revealed typical morphological features of pulmonary Langerhans cell histiocytosis (PLCH) (Figure 1A,B,C).
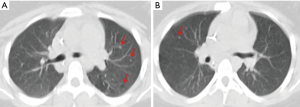
After a more comprehensive interview with family members, it was revealed that the patient had smoked 10 cigarettes/day for 4 years and CT scan revision by a thoracic expert radiologist showed small lung nodules and cystic lesions in accordance with the histological diagnosis (Figure 2A,B).
Comments
The number of lung transplants performed annually has gradually increased as well as the number of patients on the waiting list. On the other hand, the number of available organs has not had a similar trend and patients currently on the list exceed the available donors.
For this reason, many centers in the last few years have been looking for strategies to expand the donor pool, mainly related to “extended criteria” donors, with the use of EVLP (5).
EVLP allows us to evaluate organs that are not considered suitable during graft harvesting, improving some reversible alterations during normothermic perfusion, reconditioning them for a possible transplantation and overall permitting more time to carefully evaluate the donor lung (6). However, these systems are not cost effective and therefore they should be used when lungs are affected by reversible alterations, modifiable during a time frame from 4–6 to 14–15 hours. With the present case, we would like to emphasize that a careful lung evaluation is still today an important step to take also in selecting the proper case for ex vivo reconditioning. Moreover, even if the dialogue with the donor relatives is not always easy, a complete and precise history taking is essential for a more accurate assessment of donor suitability.
Pulmonary histiocytosis, also called eosinophilic granuloma of the lung, is an uncommon interstitial lung disease that is epidemiologically related to tobacco smoking. It affects young adults and primarily occurs in the third or fourth decades of life.
The most commonly reported symptoms are dyspnea, not productive cough, chest pain, fatigue, hemoptysis, and recurrent spontaneous pneumothorax (7).
HRCT of the chest is a sensitive radiological tool for the diagnosis. Radiological features include the presence of nodules, cavitary nodules and cystic lesions, alone or in combination with an upper and mid-zone predominance. It rarely shows single lesions similar to other diseases making the differential diagnosis more arduous (8). However, in our donor, at the time of evaluation a normal CT instead of HRCT was performed, without any interpretation by a dedicated pulmonary radiologist. It should also be noted that imaging techniques may be affected by changes related to prolonged hospitalization in the ICU thus there could be some difficulties in identifying this pathology by radiological images.
PLCH is histologically characterized by parenchymal infiltration of the lungs by activated Langerhans cells, macrophages, plasma cells and fibroblasts that are mainly located in the terminal and respiratory bronchioles. Fibrotic replacement of granulomatous lesions usually generates stellate scarring, with traction emphysema of adjacent alveoli. Immunohistochemical analysis is useful for the final diagnosis (S100 protein, CD1a and CD207, langerin), particularly in small specimens (7).
During retrieval, biopsy of suspicious parenchymal areas could be analyzed by frozen section analysis. However, in some nodular lesions, such as PLCH, this practice is often difficult for a precise morphological diagnosis, overall because immunohistochemistry cannot be performed in a short time.
Interestingly, however, recent evidence has shown that some biomarkers are crucial in the identification of active smokers among potential organ donors (such as a higher interleukin-8 and lower surfactant protein-D expression in BAL samples), thus this investigation could be useful in lung donor evaluation, particularly in cases where anamnestic information is missing or incomplete (9).
In the current literature there is no evidence of a PLCH (initial form), as in our case, in an accidentally implanted donor lung. However, a larger use of marginal donors, particularly with a smoking habit, puts us more on the alert for an accurate assessment during the harvesting process.
In summary, this case is exemplary to show the importance of a careful visual and tactile evaluation of donor lungs that allows us to discover an underlying lung disease. A precise collection of personal data and more in-depth evaluation of the CT scan (not necessarily HRCT) can bring about a more appropriate donor selection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: We confirm that informed consent was obtained from the family members of the patient for publication of this Case Report and any accompanying images.
References
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:1009-24. [Crossref] [PubMed]
- Tuttle-Newhall JE, Krishnan SM, Levy MF, et al. Organ donation and utilization in the United States: 1998-2007. Am J Transplant 2009;9:879-93. [Crossref] [PubMed]
- Schmack B, Weymann A, Mohite P, et al. Contemporary review of the organ care system in lung transplantation: potential advantages of a portable ex-vivo lung perfusion system. Expert Rev Med Devices 2016;13:1035-41. [Crossref] [PubMed]
- Makdisi G, Makdisi T, Jarmi T, et al. Ex vivo lung perfusion review of a revolutionary technology. Ann Transl Med 2017;5:343. [Crossref] [PubMed]
- Warnecke G, Weigmann B, Van Raemdonck D, et al. The INSPIRE International Lung Trial with the Organ Care System Technology (OCS™). J Heart Lung Transplant 2013;32:S16. [Crossref]
- Martens A, Van Raemdonck DE, Smits J, et al. A retrospective database analysis to evaluate the potential of ex vivo lung perfusion to recruit declined lung donors. Transpl Int 2017;30:1002-10. [Crossref] [PubMed]
- Roden AC, Yi ES. Pulmonary Langerhans Cell Histiocytosis: An Update From the Pathologists' Perspective. Arch Pathol Lab Med 2016;140:230-40. [Crossref] [PubMed]
- Castoldi MC, Verrioli A, De Juli E, et al. Pulmonary Langerhans cell histiocytosis: the many faces of presentation at initial CT scan. Insights Imaging 2014;5:483-92. [Crossref] [PubMed]
- Ware LB, Lee JW, Wickersham N, et al. Donor smoking is associated with pulmonary edema, inflammation and epithelial dysfunction in ex vivo human donor lungs. Am J Transplant 2014;14:2295-302. [Crossref] [PubMed]