Osimertinib therapy as first-line treatment before acquiring T790M mutation: from AURA1 trial
First-line therapy for epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC) and osimertinib
First- or second-generation EGFR tyrosine kinase inhibitors (EGFR-TKI) have been the first-line treatment for NSCLC harboring EGFR mutation (1-6), however almost all patients inevitably acquire resistance during EGFR-TKI therapy. The most common mechanism of resistance to first- or second-generation EGFR-TKIs is the T790M secondary mutation, which accounts for approximately 60% of this resistance (7,8). First- or second-generation EGFR-TKIs have either had no activity, or reduced activity for NSCLC with T790M mutation, therefore platinum-based chemotherapy had been the standard care after first- or second-generation EGFR-TKI until osimertinib appeared.
Osimertinib, a third generation irreversible EGFR-TKI with anti-tumor activity for T790M-positive NSCLC, was initially approved for patients pretreated with other EGFR-TKIs and who acquired resistance due to T790M. The first report about AZD9291, a code name of osimertinib, was opened in 2015, and the results showed good response for T790M-positive lung cancer, while showing poor response to T790M-negative lung cancer (9). The water fall plot shows the difference in response between T790M-positive and -negative, with response rates of 64% in T790M-positive and 23% in T790M-negative patients, respectively. This result impressed us that osimertinib would be the specific agent to act against T790M mutated NSCLC. We believe many doctors may have gotten a similar impression that this agent was just for second or later line therapy after a patient acquired the T790M mutation.
Phase 1 dose-escalation, and the expansion parts of the AURA (AURA1) trial evaluated the safety of osimertinib for the purpose of determining a phase 2 recommended dose. This AURA trial included two treatment-naïve cohorts, including 60 patients treated with osimertinib in first-line settings. The results of the treatment-naïve patients in AURA were reported by Ramalingam et al., indicating that osimertinib had a deep and durable response to EGFR-mutated NSCLC regardless of the presence of T790M. In this report, the overall response rate and median progression-free survival (PFS) with osimertinib, across doses were 77% (95% CI, 64–87%) and 20.5 months (95% CI, 15.0–26.1 months), respectively. The FLAURA study, a randomized phase III trial to compare the PFS of osimertinib with gefitinib, was conducted, and had been already reported (10). Consistent with the results of the AURA trial, the FLAURA trial demonstrated that osimertinib was effective regardless of acquired T790M resistance with an ORR of 80% (95% CI, 75–85%) and PFS of 18.9 months (95% CI, 15.2–21.4 months). As a result, the Food and Drug Administration (FDA) in the United States approved osimertinib for use in first-line settings for EGFR-mutant NSCLC in April 2018, which now enables the use of osimertinib without re-biopsy for detecting T790M.
The efficacy of osimertinib for a minor population: uncommon and de novo T790M
The AURA1 trial included five patients with EGFR mutation other than exon 19 deletion or exon 21 L858R point mutation, almost all of which were what we would call uncommon mutations. The median PFS of osimertinib for this population was 8.3 months (95% CI, 2.8–19.0 months). In addition, seven patients with de novo T790M were found and treated with osimertinib as a first-line treatment. With response in six of the seven patients, the response rate was 85.7%, and the duration of response (DOR) ranged from 6.9 to 27.7 months. The LUX-Lung trials previously revealed that the PFS using afatinib for patients with uncommon mutations other than T790M was 10.7 months (95% CI, 5.6–14.7 months), meanwhile the PFS for patients with both uncommon and de novo T790M mutations was 2.9 months (95% CI, 1.2–8.3 months) (11). Considering these results comprehensively, the patients with the de novo T790M mutation can be candidates for osimertinib therapy in a frontline setting, and those with uncommon mutations also could be responsive to osimertinib. For this minor population, further investigation is warranted to confirm this concept.
In the AURA1 trial, the five patients with uncommon mutations didn’t include any with EGFR exon 20 insertions, and 4 of the 5 patients had the G719X mutation. The LUX-Lung trial showed the ineffectiveness of afatinib for patients with tumors harboring exon 20 insertions with this group having the shortest PFS compared with the chemotherapy groups. (9.2 vs. 30.2 months). These findings indicated that afatinib is inefficacious for exon 20 insertion-mutant NSCLC, and first-generation EGFR-TKIs also were reported to have poor activity for exon 20 insertions mutation (12). On the other hand, osimertinib showed potent activity against the exon 20 insertions mutant cell line in vitro (13). A single-arm phase 2 trial to assess the efficacy of osimertinib for exon 20 insertion-mutant NSCLC is ongoing in Korea (NCT03414814), which is expected to confirm the efficacy of osimertinib for this patient class.
Comparative analysis of osimertinib across doses between 80 and 160 mg
In addition to the effectiveness of osimertinib as a first-line therapy, the AURA1 study provided us some indications with clinical interest regarding the control of adverse events.
This report was the only one which provided us with data about osimertinib therapy using a dose of 160 mg once daily. From the results of this phase I trial, the recommended dose of osimertinib in further trials for first- or second-line settings was 80 mg once daily. As we see from the safety profile in AURA1, there was no significant difference in the occurrence rate of adverse events of grade 3 or more between the 80 mg and the 160 mg dosage groups (60% vs. 63%). The dose of 160 mg, however, had a higher rate of reduction of osimertinib compared to the dose of 80 mg (53% vs. 10%), and there is no significant difference in PFS between two arms (22.1 in 80-mg vs. 19.3 in 160-mg, months), resulting in 80 mg being the recommended dose for the further clinical trials.
Comparing the adverse events between the 80-mg and 160-mg dosage groups in the data supplement, the 160-mg dosage increased the occurrence rate of some adverse events: rash (73% vs. 87%), diarrhea (60% vs. 87%), paronychia (40% vs. 70%), white blood cell decrease (13% vs. 27%). These adverse events can be dose-dependent, suggesting that a dose reduction can be effective for patients who experience the adverse events listed above. In clinical practice, osimertinib is permitted to be decreased to 40 mg once daily.
The mechanisms of resistance to osimertinib in AURA1
Ramalingam et al. reported translational research in the AURA1 trial for detecting the mechanisms of resistance to osimertinib using plasma samples before or after osimertinib therapy. In the results of the translational research, the list of the detected mechanisms of resistance to osimertinib were described in the data supplement. The list included two patients with C797S, two of KRAS, one of MET, JAK2, and HER2 mutation, with some patients having multiple mutations.
In clinical practice, clarifying the mechanisms of resistance to osimertinib is crucial for physicians considering second-line treatment. The effective treatment after osimertinib failure remains unclear, although osimertinib in second-line settings has become the established protocol for the treatment of lung cancer with acquired resistance due to T790M mutation after first-generation EGFR-TKI failure.
A recent report demonstrated the detected mechanism of resistance to ALK-TKI depended on the concentration of exposure to prior TKI (14). Afatinib was reported to induce the C797S mutation with low-doses (15). These results showed the concentration of exposure to TKI could influence the detected mechanism of resistance. Unfortunately, the correlation between the dose of osimertinib and resistance could not be analyzed from the data of the AURA1 trial because the information about the dose of osimertinib for each patient with resistance to osimertinib was not described in this report. High-dose osimertinib would suppress some of the resistance which was detected after low-dose osimertinib exposure, which is required to be investigated in the future.
A previous report indicated that C797S seems to be a common secondary mutation for resistance to osimertinib, and accounts for approximately 20% in patients who experienced disease progression during osimertinib therapy (16). The quinazoline-based EGFR-TKI, gefitinib, has been shown to have activity for tumors with C797S mutation (17). Moreover, the fourth-generation EGFR-TKI, EAI045, which has shown to effectively inhibit the proliferation of cell lines with C797S mutation, has been recently improved (18). These agents will be considered as one of the treatment options when detecting C797S after osimertinib therapy failure.
The efficacy of osimertinib for central nervous system (CNS) metastasis
In the field of NSCLC, metastasis of the CNS has been the highest interest issue to address for clinical physicians. Fifteen patients with asymptomatic or stable CNS metastasis were enrolled in the AURA1 trial. Although information about the response to osimertinib of patients with CNS metastasis in AURA1 was not described in the article, the rate of CNS progression in the patients who continued osimertinib therapy beyond the point of disease progression was described in the data supplement. The rates of progression among the CNS patients were 15% and 6% in the 80-mg group and 160-mg group respectively, and the occurrence rate of new lesion of CNS were 15% and 0%, respectively.
Consistent with these results of the AURA1 trial, other clinical trials showed the clinical benefit of osimertinib for CNS metastasis: AURA2 showed that 58 of 84 patients with CNS metastasis had a response to osimertinib (19), AURA3 revealed that patients with CNS metastases had significantly longer PFS in the osimertinib arm compared with the platinum-pemetrexed arm [8.5 months in osimertinib vs. 4.2 months in platinum-pemetrexed, months; HR, 0.32 (95% CI, 0.21–0.49) (20), and the trend was confirmed in first-line settings in the FLAURA trial (PFS, 15.2 months in osimertinib vs. 9.6 months in standard EGFR-TKI; HR, 0.47 (95% CI, 0.30–0.74)] (10).
From the series of these results, there is no doubt whatsoever about the clinical benefit of osimertinib for patients with CNS metastasis, however, whether initial radiotherapy should be conducted before osimertinib therapy, stereotactic radiosurgery (SRS) or whole brain radiation therapy (WBRT), remains an unsolved clinical question.
The future prospects of osimertinib as a first-line treatment
The AURA1 trial provided us with valuable information about the safety profile of different doses of osimertinib, the mechanisms of resistance to osimertinib, and the efficacy for uncommon or de novo T790M mutations, all of which cannot be assessed in the other clinical trials. From these findings, we were able to predict appropriate patients for osimertinib therapy, and some prospective trials for this population are already in progress at present.
Osimertinib was proven to prolong post-progression survival after gefitinib or erlotinib failure in the AURA3 trial, but a treatment which prolongs PPS after osimertinib failure has not yet been found.
Retrospective analysis of the LUX-Lung 3, 6 and 7 trials showed that the sequential therapy of afatinib followed by osimertinib had longer overall survival compared with that of gefitinib followed by osimertinib (21). In addition, the PFS of dacomitinib, a novel second-generation irreversible EGFR-TKI, was 14.7 months in the ARCHER 1050 trial (22). Considering the PFS of dacomitinib in the ARCHER 1050 trial, and the PFS of osimertinib in the AURA3 trial (dacomitinib, 14.7 months; osimertinib in AURA3, 10.1 months), 24.8 months of total PFS is longer than the PFS of osimertinib used as a first-line therapy in the FLAURA trial of 18.9 months.
Indeed, there are some limitations in the above consideration; the ACHER 1050 trial excluded patients with CNS metastasis, and the AURA3 trial proved the efficacy of osimertinib after first generation EGFR-TKI failure, but not after second-generation EGFR-TKI failure. This suggests that the matured data of overall survival in the FLAURA trial should be considered to discuss an appropriate first-line EGFR-TKI.
The APPLE study, a phase 2 trial to compare the clinical benefit among three arms, osimertinib as first-line in arm A, gefitinib followed by osimertinib based on T790M-positive by cell-free DNA (cfDNA) in arm B, and RECIST PD in arm C, with a primary endpoint of PFS rate at 18 months, is ongoing to investigate the best sequential strategy of EGFR-TKI for EGFR-mutant NSCLC (23).
In addition, the combination therapy of erlotinib plus bevacizumab or gefitinib plus chemotherapy were reported in 2018, consequently the option of first-line therapy for EGFR-mutated NSCLC increased (Table 1).
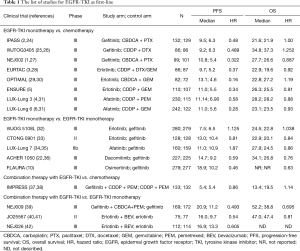
Full table
The result of future trials, the data from clinical practice, and the investigation into the mechanisms of resistance to osimertinib will provide us with a clue for the frontline treatment strategy of EGFR-mutant NSCLC.
Acknowledgements
This article was revised in English by Shane LeGros.
Footnote
Conflicts of Interest: Honoraria: Boehringer Ingelheim, AstraZeneca, Chugai Pharmaceutical Co, MSD, Ono Pharmaceutical Co, Eli Lilly, Shionogi Pharmaceutical Co; Research funding: GlaxoSmithKlein, Novartis, Daiichi-Sankyo, Boehringer Ingelheim, AstraZeneca, Chugai Pharmaceutical Co, Ono Pharmaceutical Co, Kyorin Pharmaceutical Co.
References
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883-9. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. [Crossref] [PubMed]
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616-22. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. [Crossref] [PubMed]
- Naidoo J, Sima CS, Rodriguez K, et al. Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: Clinical outcomes and response to erlotinib. Cancer 2015;121:3212-20. [Crossref] [PubMed]
- Floc'h N, Martin MJ, Riess JW, et al. Antitumor Activity of Osimertinib, an Irreversible Mutant-Selective EGFR Tyrosine Kinase Inhibitor, in NSCLC Harboring EGFR Exon 20 Insertions. Mol Cancer Ther 2018;17:885-96. [Crossref] [PubMed]
- Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov 2018;8:714-29. [Crossref] [PubMed]
- Kobayashi Y, Azuma K, Nagai H, et al. Characterization of EGFR T790M, L792F, and C797S Mutations as Mechanisms of Acquired Resistance to Afatinib in Lung Cancer. Mol Cancer Ther 2017;16:357-64. [Crossref] [PubMed]
- Sullivan I, Planchard D. Osimertinib in the treatment of patients with epidermal growth factor receptor T790M mutation-positive metastatic non-small cell lung cancer: clinical trial evidence and experience. Ther Adv Respir Dis 2016;10:549-65. [Crossref] [PubMed]
- Ercan D, Choi HG, Yun CH, et al. EGFR Mutations and Resistance to Irreversible Pyrimidine-Based EGFR Inhibitors. Clin Cancer Res 2015;21:3913-23. [Crossref] [PubMed]
- Jia Y, Yun CH, Park E, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature 2016;534:129-32. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Sequist L, Wu YL, Schuler M, et al. Subsequent therapies post-afatinib among patients (pts) with EGFR mutation-positive (EGFRm+) NSCLC in LUX-Lung (LL) 3, 6 and 7. Ann Oncol 2017;28. [Crossref]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR -mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Remon J, Menis J, Hasan B, et al. The APPLE Trial: Feasibility and Activity of AZD9291 (Osimertinib) Treatment on Positive PLasma T790M in EGFR-mutant NSCLC Patients. EORTC 1613. Clin Lung Cancer 2017;18:583-8. [Crossref] [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Yoshioka H, Mitsudomi T, Morita S, et al. Final overall survival results of WJTOG 3405, a randomized phase 3 trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer (NSCLC) harboring mutations of the epidermal growth factor receptor (EGFR). J Clin Oncol 2014;32:8117.
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54-9. [Crossref] [PubMed]
- Leon LF, Golsorkhi A, Liu S, et al. 1273P Overall survival analyses of first-line erlotinib versus chemotherapy in the EURTAC study population controlling for the use of post-study therapy. Ann Oncol 2014;25:iv447-8. [Crossref]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877-83. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Urata Y, Katakami N, Morita S, et al. Randomized Phase III Study Comparing Gefitinib with Erlotinib in Patients with Previously Treated Advanced Lung Adenocarcinoma: WJOG 5108L. J Clin Oncol 2016;34:3248-57. [Crossref] [PubMed]
- Kim ST, Uhm JE, Lee J, et al. Randomized phase II study of gefitinib versus erlotinib in patients with advanced non-small cell lung cancer who failed previous chemotherapy. Lung Cancer 2012;75:82-8. [Crossref] [PubMed]
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. [Crossref] [PubMed]
- Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270-7. [Crossref] [PubMed]
- Mok TS, Cheng Y, Zhou X, et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients with Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J Clin Oncol 2018;36:2244-50. [PubMed]
- Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol 2015;16:990-8. [Crossref] [PubMed]
- Mok TSK, Kim SW, Wu YL, et al. Gefitinib Plus Chemotherapy Versus Chemotherapy in Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer Resistant to First-Line Gefitinib (IMPRESS): Overall Survival and Biomarker Analyses. J Clin Oncol 2017;35:4027-34. [Crossref] [PubMed]
- Nakamura A, Inoue A, Morita S, et al. Phase III study comparing gefitinib monotherapy (G) to combination therapy with gefitinib, carboplatin, and pemetrexed (GCP) for untreated patients (pts) with advanced non-small cell lung cancer (NSCLC) with EGFR mutations (NEJ009). J Clin Oncol 2018;36: abstr 9005.
- Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236-44. [Crossref] [PubMed]
- Yamamoto N, Seto T, Nishio M, et al. Erlotinib plus bevacizumab (EB) versus erlotinib alone (E) as first-line treatment for advanced EGFR mutation–positive non-squamous non–small-cell lung cancer (NSCLC): Survival follow-up results of JO25567. J Clin Oncol 2018;36:abstr 9007.
- Furuya N, Fukuhara T, Saito H, et al. Phase III study comparing bevacizumab plus erlotinib to erlotinib in patients with untreated NSCLC harboring activating EGFR mutations: NEJ026. J Clin Oncol 2018;36:abstr 9006.


