Donor selection in heart transplantation
Introduction
Despite the rapid growth of ventricular assist devices in heart failure, heart transplantation remains the gold standard for long term outcomes in patients with medically refractory heart failure. The shortcoming in transplantation remains the relatively stable organ supply in the face of rising organ demands. In the United States, the number of heart transplants being performed over the past two decades has remained steady between 2,000 to 2,500 being performed annually. The lack of readily available organs in addition to increased scrutiny over quality and outcomes in health care, has led the Centers for Medicare and Medicaid Services (CMS) to raise the standards for individual institutional outcomes to match national mortality and graft survival outcomes. An important component of outcomes and graft survival is the decision of which organs are suitable as donor organs for transplantation. Appropriate donor selection and management has become paramount in maintaining and optimizing outcomes following heart transplantation.
Donor selection logistics overview
Patients with end stage heart failure who are approved as transplant candidates and listed by the criteria outlined by the United Network for Organ Sharing (UNOS) are eligible for being recipients of an appropriate donor heart (see Table 1). In most transplant centers the process is started by the collaboration between the institutional transplant coordinator and the local organ procurement organization. Potential heart donors are identified and a preliminary matching list generated based on UNOS criteria. The primary survey of the donor includes the confirmation of brain death, verification of consent for donation, ABO blood typing, demographics, identification of potential co-morbid conditions (including high risk behavior, substance abuse history, mechanism of death) and the need for cardiopulmonary resuscitation (and if so duration from initiation to return of vital signs). A more heart specific assessment includes the requirement of inotropic support, hemodynamic stability, presence of thoracic trauma, serum cardiac enzyme markers [troponin, or if troponin not available creatinine phosphokinase (CPK)-MB fraction], electrocardiogram, echocardiogram and coronary angiography when indicated (presence of co-morbid conditions and/or age) (1). After a full on-site review of pertinent hospital records, the hemodynamic performance of the heart (including right and left heart catheterization data), visual and manual inspection of the heart, the final acceptance of the heart for transplantation is made by the procuring cardiothoracic surgeon.

Full table
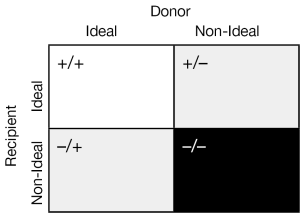
The two central and unifying concepts in the selection of a donor heart for transplantation are (I) the quality of the donor heart and (II) the matching of the donor heart to the recipient’s individual needs. The standard criteria used to accept donor hearts are summarized in Table 2. There are institutional as well as individual recipient demand exceptions to these criteria and a certain “art” of balancing recipient need with donor availability. Indeed it may be helpful to think of the matching of the donor heart to the recipient as follows (See Figure 1).

Full table
Quality assessment of donor heart
The major components for the assessment of the donor heart centers around a thorough understanding of the donor history, physical examination, hemodynamic evaluation in addition to laboratory and radiographical (echocardiogram, possible cardiac angiography) findings.
Age
The importance of the age of the donor heart can be traced to early reports on recommendations for heart transplantation. Indeed, an early conservative age for the upper limits of acceptable organs was 35 years of age (2). This has been gradually increased over the past several decades, with most centers now using donor age <55 years as a cut off with the most liberal center using donors up to age 65 and greater. Despite the increase in the upper limits of acceptable age, more than 50% of adult heart donors remain between the ages of 18-34 in the UNOS database with a relatively fixed percentage during the time period of 1988 to 2013.
Multiple studies looking at various recipient and donor factors have shown that age is an independent risk factor for long term mortality. One study looking at the UNOS database with pre-transplant donor and recipient data that broke down the donor age by decades showed an increased odds ratio for mortality based on donor age 50-59 years old: OR 1.8 (1.4-2.0); 40-49 years old: OR 1.7 (1.3-1.7); 30-39 years old: 1.3 (1.1-1.5) all with P<0.05 (3). Other single institutional studies have shown a correlate between early graft failure or patient mortality with the combination of both recipient and donor age >60 (4).
Function of donor heart
It is relatively common for potential donor hearts that have either undergone cardiopulmonary resuscitation, a neurologic insult, thoracic trauma or are on vasoactive/inotropic agents to display non-specific ST changes on electrocardiogram and/or have elevated CPK-MB or troponin levels. Although it has been shown that modestly elevated donor cardiac troponin I levels do not have a negative influence in post-transplant mortality or need for mechanical circulatory support (5), it is important to correlate the findings with echocardiographic examination. In interpretation of the echocardiogram findings; however, it is important to keep in mind the time period between the inciting event, possible myocardial stunning and recovery.
All potential donors should undergo a full echocardiographic examination and it can be argued that this is the single most important tool for examination of donor heart function (6). There should be particular attention paid to the presence of left ventricular hypertrophy (LVH), significant physiologic valvular dysfunction, and depressed ventricular function. A retrospective, single institutional study out of Stanford concentrating on LVH showed decreased survival in heart transplant recipients whose donor heart left ventricular wall thickness exceeded 1.4 mm (7). This underscores the need for a careful echocardiographic examination in any donor with significant age (>40 years old), history of hypertension, substance abuse or risk factors for coronary arterial disease.
Additionally, the need for either inotropic or vasopressor support should be noted. It is important to differentiate between inotropic support secondary to poor cardiac output and vasopressor support secondary to peripheral vasoplegia. Although it is common to need either inotropic or vasopressor support, caution should be used in older donors who may have risk factors for coronary arterial disease, hypertension or left ventricular hypertrophy as stated above. A multi-institutional retrospective study of 512 patients showed that the donor use of norepinephrine infusion did not negatively affect early survival (8). Indeed, an often quoted study out of Papworth Hospital showed an increased donor yield by continuously monitoring hemodynamic donor data prior to organ procurement. The study consisted of using two sets of hemodynamic data—at initial assessment and just before organ procurement. Donors were subdivided into category A (good function throughout), category B (sub-optimal function then improvement) and category C (decreasing or poor function throughout). Although organs used from categories B and C did not compromise 30 days or 1 year mortality, the authors warned of using these organs in combination with other risk factors (such as older age and longer ischemic times) (9). This underscores the need for initial and continuous evaluation of the potential donor heart during the placement process and how an organized strategy can increase donor usage.
In our institution, we reserve coronary angiography for donor hearts >40 years of age or with significant risk factors (hypertension, diabetes mellitus, hyperlipidemia, family history, smoking or concerning findings on echocardiogram). The presence of coronary arterial disease in the donor heart, as well as increased donor age, been correlated with coronary allograft vasculopathy (10). Although it is our policy not to use donors with multi-vessel coronary arterial disease for transplantation at our institution, several centers have reported with modest success in the use of single- or two-vessel effected donor hearts (11-13).
Decision on appropriateness of heart for recipient
A successful heart transplantation goes beyond just having a perfect donor organ. There are a multitude of other components to the equation including ischemic time, recipient co-morbidities and condition at time of transplantation, size matching, presence of panel reactive antibodies (PRAs) that must all be accounted for to optimize chance of success.
Donor—recipient compatibility
Recently, literature on gender matching of donor to recipient (both without previous sternotomy and with LVADs at bridge to transplant) has shown improved graft survival after transplantation in donor-recipient concordance (14,15). The downside of gender mismatch is observed more in male recipients from female donors and is correlated with both frequency and severity of graft rejection (16). Along those lines, size matching between donor and recipient deserves special mention. The caution of placing a small donor heart size relative to the recipient is warranted; however, size matching based on either body mass index or height may be more precise than weight alone. Extra caution must be exercised not to undersize the donor heart size to the recipient by more than 30% mismatch in patients with known pulmonary hypertension. Additionally, there should be hesitation to oversize by more than 30% mismatch in any recipient who has had a recent large myocardial infarction, LVAD placement or previous sternotomies as the pericardial space may prove to be restrictive.
Ischemic time
Currently, an ischemic time of less than four hours is optimal with some centers showing acceptable outcomes with longer ischemia times (17). There are however, many reports showing that longer ischemia times are associated with higher risk of mortality (3,15,18). In fact, in a study utilizing the UNOS database of over 11,700 patients undergoing heart transplantation, the ischemia time was shown to be an independent risk factor for survival with an OR of 1.7 (1.0-2.8) in patients with an ischemic time >6 hours and an OR of 1.4 (1.3-1.6) in patients with an ischemic time between 4-6 hours (P<0.05 for both) (3).
Expanding the donor criteria
The fixed supply of donor hearts with an increasing demand by patients with heart failure, have made the increasing use of available hearts as suitable donor organs a priority. In 2001, a concerted effort to maximize use of organs recovered from the deceased donor was outlined as the Crystal City guidelines (see Figure 2) (19). This was in direct response to the mortality of nearly 17% per year while waiting on the transplant list combined with the 42% donor yield based on the UNOS data in 1998. This has indeed born out in other studies as well. In a study looking at 1,872 potential donors in California from 2001-2008, only 45% of organs were used. Among the various reasons listed for not using the available organs were age >50 years, female sex, death from cerebrovascular accident, hypertension, diabetes mellitus, left ventricular dysfunction, wall motion abnormalities and elevated troponin levels. However, the only thing shown on further analysis to increase recipient mortality on the hearts that were used was the presence of diabetes mellitus in the donor organ (20). The presence of insulin dependent donors as an independent risk factor for mortality was also found in the analysis of the UNOS database with an OR of 1.8 (1.0-3.2), P<0.05 (21).
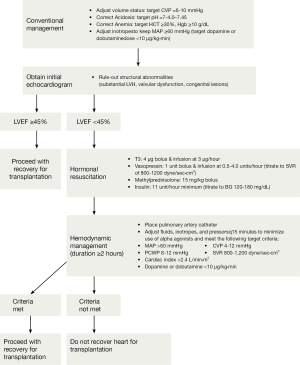
Additionally, that same study showed that hepatitis C (+) donors had an OR 2.2 (1.1-4.0 CI) for mortality, P<0.05. This has led some to totally abandon the use of high risk social behavior patients (incarceration, unprofessional tattoos, alternative life style practice, active oral or intravenous substance abuse) in heart transplantation and others to be highly selective in their use (22). Interestingly, a recent study of UNOS database showed donor cocaine use did not alter mortality or development of coronary allograft vasculopathy in the first one or five years post-transplantation (23). Some centers have even transplanted recipients with known human immunodeficiency virus (24,25).
A question that has arisen recently is whether or not one can either optimize, repair or recover a potential heart donor to make it suitable for organ transplantation.
Successful use of stress echocardiography to show contractile reserve in donors with low ejection fraction has led to six patients at a single institution being transplanted uneventfully (26). This has previously been demonstrated in a larger, but younger cohort of donors (27). The concept of potential repair of a less than perfect organ is particularly attractive in expanding the donor pool from donation after cardiac death donors. Although there are encouraging reports of no difference in five years mortality and graft survival rates, this has yet to become mainstream (28). Akin to ex vivo lung perfusion, donation after cardiac death donors would be a great platform for testing the concept of repair by ex vivo heart perfusion (29). An additional benefit would be the ability to perform invasive angiography without the logistical pitfalls of donor transportation from donor institutions that currently lack immediate access to coronary angiography (30).
Special considerations
There is an increasing role of recipient-donor matching in transplantation as it relates to circulating antibodies against human leukocyte antigens and nonhuman leukocyte antigens—or allosensitization. Although there is some dispute as to efficacy of desensitization in post-transplant outcomes, the rise of bridging patients to transplantation with ventricular assist devices and thus exposure to prior allosensitization has thrust this issue to the forefront (31-33). Patients who have allosensitization have a decreased possible donor pool, longer time to transplant and poorer survival (31,34). As a result of this, panel reactive antibodies (PRA) are routinely tested. Traditionally a complement-dependent cytotoxicity assay was used with newer methods of flow cytometry, ELISA and most recently Luminex testing being employed for donor-patient specific crossmatching to ensure optimal transplant outcomes (35). Depending on recipient stability and geographic location, three ways to perform crossmatching are in a prospective, retrospective or virtual manner. Prospective crossmatching involves matching the donor with the recipient by directly testing blood and although ideal, is geographically and logistically challenging (36). Many institutions, including ours, have employed various desensitization protocols to reduce the levels of PRA including intravenous immune globulin, plasmapheresis, rituximab or cyclophosphamide (or a combination) to allow for a bigger donation pool for our recipients (37,38). Some institutions have gone so far as to perform plasmapheresis and alemtuzumab during the cardiopulmonary bypass run in LVAD patients with high PRAs at time of heart transplantation (39). More standard techniques when prospective crossmatching is not available are to either perform the crossmatch in a retrospective or virtual manner. Retrospective crossmatching involves direct comparison of the donor and recipient blood but with the results being available after the donor heart has been used for transplantation. Virtual crossmatch involves comparing the recipients specific PRAs in the past with the donors blood and making decisions based on an indirect comparison. Each of these techniques is appropriate in various clinical scenarios and has decreased the chance of primary graft dysfunction and rejection. Unfortunately, this has come at the price of increased wait list times for recipients with high PRAs.
Some institutions have begun an extended criterion—alternate list for high risk heart transplant recipients. This has allowed the use of marginal donor organs in a recipient cohort that is sicker and without much alternatives or physiologic reserve (40,41). In a study from Duke University, patients transplanted from the alternative list were compared to patients with ventricular assist device as destination therapy. Although survival rates were similar after one year (82% for transplanted group vs. 78% for LVAD group), the transplanted group had a trend towards, but not statistically significant, better three years survival (64% versus 50%, P=0.33) (34).
Conclusions
In this current era of transplantation, there is increased focus on outcomes as it relates to volume and quality (42). Although we are well aware that institutional volume is not a surrogate for center quality, we must resist the temptation to be risk-averse and deny patients the chance at receiving lifesaving organs (43). In order to best accomplish this, it is imperative to have a better understanding of donor risk factors that can affect graft and patient survival (3,44,45). The continued increase in LVAD usage combined with the discrepancy between donor organ supply and demand means getting the most out of the organs that are used. Additionally, there should be a concerted effort between organ procurement organizations, transplant programs and donor hospitals to maximize the utilization of marginal donor hearts.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- John R. Donor management and selection for heart transplantation. Semin Thorac Cardiovasc Surg 2004;16:364-9. [PubMed]
- Griepp RB, Stinson EB, Clark DA, et al. The cardiac donor. Surg Gynecol Obstet 1971;133:792-8. [PubMed]
- Hong KN, Iribarne A, Worku B, et al. Who is the high-risk recipient? Predicting mortality after heart transplant using pretransplant donor and recipient risk factors. Ann Thorac Surg 2011;92:520-7; discussion 527. [PubMed]
- Cannata A, Botta L, Colombo T, et al. Does the cardioplegic solution have an effect on early outcomes following heart transplantation? Eur J Cardiothorac Surg 2012;41:e48-52; discussion e52-3.
- Khush KK, Menza RL, Babcock WD, et al. Donor cardiac troponin I levels do not predict recipient survival after cardiac transplantation. J Heart Lung Transplant 2007;26:1048-53. [PubMed]
- Hashimoto S, Kato TS, Komamura K, et al. Utility of echocardiographic evaluation of donor hearts upon the organ procurement for heart transplantation. J Cardiol 2011;57:215-22. [PubMed]
- Kuppahally SS, Valantine HA, Weisshaar D, et al. Outcome in cardiac recipients of donor hearts with increased left ventricular wall thickness. Am J Transplant 2007;7:2388-95. [PubMed]
- Fiorelli AI, Branco JN, Dinkhuysen JJ, et al. Risk factor analysis of late survival after heart transplantation according to donor profile: a multi-institutional retrospective study of 512 transplants. Transplant Proc 2012;44:2469-72. [PubMed]
- Stoica SC, Satchithananda DK, Charman S, et al. Swan-Ganz catheter assessment of donor hearts: outcome of organs with borderline hemodynamics. J Heart Lung Transplant 2002;21:615-22. [PubMed]
- Gao HZ, Hunt SA, Alderman EL, et al. Relation of donor age and preexisting coronary artery disease on angiography and intracoronary ultrasound to later development of accelerated allograft coronary artery disease. J Am Coll Cardiol 1997;29:623-9. [PubMed]
- Pinto CS, Prieto D, Antunes MJ. Coronary artery bypass graft surgery during heart transplantation. Interact Cardiovasc Thorac Surg 2013;16:224-5. [PubMed]
- Grauhan O, Siniawski H, Dandel M, et al. Coronary atherosclerosis of the donor heart--impact on early graft failure. Eur J Cardiothorac Surg 2007;32:634-8. [PubMed]
- Marelli D, Laks H, Bresson S, et al. Results after transplantation using donor hearts with preexisting coronary artery disease. J Thorac Cardiovasc Surg 2003;126:821-5. [PubMed]
- Maltais S, Jaik NP, Feurer ID, et al. Mechanical circulatory support and heart transplantation: donor and recipient factors influencing graft survival. Ann Thorac Surg 2013;96:1252-8. [PubMed]
- Kilic A, Weiss ES, Allen JG, et al. Simple score to assess the risk of rejection after orthotopic heart transplantation. Circulation 2012;125:3013-21. [PubMed]
- Welp H, Spieker T, Erren M, et al. Sex mismatch in heart transplantation is associated with increased number of severe rejection episodes and shorter long-term survival. Transplant Proc 2009;41:2579-84. [PubMed]
- Morgan JA, John R, Weinberg AD, et al. Prolonged donor ischemic time does not adversely affect long-term survival in adult patients undergoing cardiac transplantation. J Thorac Cardiovasc Surg 2003;126:1624-33. [PubMed]
- Bourge RC, Naftel DC, Costanzo-Nordin MR, et al. Pretransplantation risk factors for death after heart transplantation: a multiinstitutional study. The Transplant Cardiologists Research Database Group. J Heart Lung Transplant 1993;12:549-62. [PubMed]
- Zaroff JG, Rosengard BR, Armstrong WF, et al. Consensus conference report: maximizing use of organs recovered from the cadaver donor: cardiac recommendations, March 28-29, 2001, Crystal City, Va. Circulation 2002;106:836-41.
- Khush KK, Menza R, Nguyen J, et al. Donor predictors of allograft use and recipient outcomes after heart transplantation. Circ Heart Fail 2013;6:300-9. [PubMed]
- Hong KN, Iribarne A, Worku B, et al. Who is the high-risk recipient? Predicting mortality after heart transplant using pretransplant donor and recipient risk factors. Ann Thorac Surg 2011;92:520-7; discussion 527. [PubMed]
- Xu DS, Hartman D, Ludrosky K, et al. Impact of donor high-risk social behaviors on recipient survival in cardiac transplantation. Transplantation 2010;89:873-8. [PubMed]
- Brieke A, Krishnamani R, Rocha MJ, et al. Influence of donor cocaine use on outcome after cardiac transplantation: analysis of the United Network for Organ Sharing Thoracic Registry. J Heart Lung Transplant 2008;27:1350-2. [PubMed]
- Sims DB, Uriel N, González-Costello J, et al. Human immunodeficiency virus infection and left ventricular assist devices: a case series. J Heart Lung Transplant 2011;30:1060-4. [PubMed]
- Krishan K, Pinney S, Anyanwu AC. Successful left ventricular assist device bridge to transplantation in a patient with end-stage heart failure and human immunodeficiency virus. Artif Organs 2012;36:759. [PubMed]
- Bombardini T, Gherardi S, Leone O, et al. Transplant of stunned donor hearts rescued by pharmacological stress echocardiography: a “proof of concept” report. Cardiovasc Ultrasound 2013;11:27. [PubMed]
- Kono T, Nishina T, Morita H, et al. Usefulness of low-dose dobutamine stress echocardiography for evaluating reversibility of brain death-induced myocardial dysfunction. Am J Cardiol 1999;84:578-82. [PubMed]
- Quader MA, Wolfe LG, Kasirajan V. Heart transplantation outcomes from cardiac arrest-resuscitated donors. J Heart Lung Transplant 2013;32:1090-5. [PubMed]
- White CW, Ali A, Hasanally D, et al. A cardioprotective preservation strategy employing ex vivo heart perfusion facilitates successful transplant of donor hearts after cardiocirculatory death. J Heart Lung Transplant 2013;32:734-43. [PubMed]
- Ghodsizad A, Bordel V, Ungerer M, et al. Ex vivo coronary angiography of a donor heart in the organ care system. Heart Surg Forum 2012;15:E161-3. [PubMed]
- Al-Mohaissen MA, Virani SA. Allosensitization in heart transplantation: an overview. Can J Cardiol 2014;30:161-72. [PubMed]
- Shankar N, Daly R, Geske J, et al. LVAD implant as a bridge to heart transplantation is associated with allosensitization as measured by single antigen bead assay. Transplantation 2013;96:324-30. [PubMed]
- Kilic A, Ailawadi G. Left ventricular assist devices in heart failure. Expert Rev Cardiovasc Ther 2012;10:649-56. [PubMed]
- Wright EJ, Fiser WP, Edens RE, et al. Cardiac transplant outcomes in pediatric patients with pre-formed anti-human leukocyte antigen antibodies and/or positive retrospective crossmatch. J Heart Lung Transplant 2007;26:1163-9. [PubMed]
- Chaidaroglou A, Skoura A, Degiannis D. Comparative performance evaluation of a donor-specific bead-based crossmatch technique for the detection of donor-specific anti-HLA antibodies in heart transplantation. Transplant Proc 2013;45:2005-8. [PubMed]
- Patel JK, Kobashigawa JA. Improving survival during heart transplantation: diagnosis of antibody-mediated rejection and techniques for the prevention of graft injury. Future Cardiol 2012;8:623-35. [PubMed]
- Ballew CC, Bergin JD. Management of patients with preformed reactive antibodies who are awaiting cardiac transplantation. Am J Crit Care 2005;14:46-51. [PubMed]
- Chih S, Tinckam KJ, Ross HJ. A survey of current practice for antibody-mediated rejection in heart transplantation. Am J Transplant 2013;13:1069-74. [PubMed]
- Lick SD, Beckles DL, Piovesana G, et al. Transplantation of high panel-reactive antibody left ventricular assist device patients without crossmatch using on-bypass pheresis and alemtuzumab. Ann Thorac Surg 2011;92:1428-34. [PubMed]
- Laks H, Marelli D. The alternate recipient list for heart transplantation: a model for expansion of the donor pool. Adv Card Surg 1999;11:233-44. [PubMed]
- Daneshmand MA, Rajagopal K, Lima B, et al. Left ventricular assist device destination therapy versus extended criteria cardiac transplant. Ann Thorac Surg 2010;89:1205-9; discussion 1210. [PubMed]
- Abecassis MM, Burke R, Klintmalm GB, et al. American Society of Transplant Surgeons transplant center outcomes requirements--a threat to innovation. Am J Transplant 2009;9:1279-86. [PubMed]
- Kilic A, Weiss ES, Yuh DD, et al. Institutional factors beyond procedural volume significantly impact center variability in outcomes after orthotopic heart transplantation. Ann Surg 2012;256:616-23. [PubMed]
- Weiss ES, Allen JG, Kilic A, et al. Development of a quantitative donor risk index to predict short-term mortality in orthotopic heart transplantation. J Heart Lung Transplant 2012;31:266-73. [PubMed]
- Weiss ES, Allen JG, Arnaoutakis GJ, et al. Creation of a quantitative recipient risk index for mortality prediction after cardiac transplantation (IMPACT). Ann Thorac Surg 2011;92:914-21; discussion 921-2. [PubMed]


