Combination immuno-oncology therapy with pembrolizumab, an anti-PD-1 monoclonal antibody targeting immune evasion, and standard chemotherapy for patients with the squamous and non-squamous subtypes of non-small cell lung cancer
Immuno-oncology drugs that inhibit immunosuppressive receptors (CTLA4, LAG3, PD-1, TIGIT and TIM3) or activate immunostimulatory receptors (4-1BB, GITR, ICOS and OX40) are emerging as promising therapeutics for cancer patients (1-3). PD-1 (CD279 or PDCD1), consisting of the extracellular N-terminal loop and immunoglobulin variable (IgV) domain, a single transmembrane domain and intracellular ITIM and ITSM motifs, is a representative target of immuno-oncology therapy (4-6). PD-L1 and PD-L2 ligands on tumor cells or tissue macrophages bind to the PD-1 receptor on T cells to induce the phosphorylation of ITIM (Y223) and ITSM (Y248) motifs of PD-1 and the subsequent repression of PI3K and PLCγ1 signaling cascades (Figure 1). Because ligand-dependent PD-1 signaling activation leads to immune evasion through the suppression of anti-tumor immunity in the tumor microenvironment, anti-PD-1 monoclonal antibodies (mAbs) [camrelizumab/SHR-1210 (7), cemiplimab/REGN2810 (8), MEDI0680/AMP-514 (9), nivolumab (10), pembrolizumab (11), sintilimab/IBI308 (12) and tislelizumab/BGB-A317 (13)] and anti-PD-L1 mAbs [atezolizumab (14), avelumab (15), BMS-936559 (16) and durvalumab (17)] have been developed as investigational drugs targeting immune evasion, also known as immune checkpoint blockers.
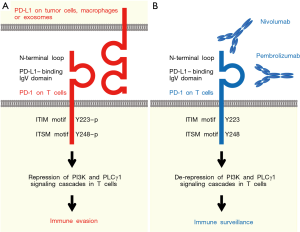
Among these anti-PD-1 mAbs, nivolumab and pembrolizumab have been approved by the US Food and Drug Administration (FDA) for the treatment of cancer patients: nivolumab for classical Hodgkin lymphoma, colorectal cancer, hepatocellular carcinoma, head and neck squamous cell carcinoma (HNSCC), melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma and urothelial carcinoma; whereas pembrolizumab for a relatively wider range of cancers, including cervical cancer, gastric cancer, HNSCC, Hodgkin lymphoma, melanoma, NSCLC, primary mediastinal large B-cell lymphoma, urothelial carcinoma and microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) cancers (https://www.cancer.gov/about-cancer/treatment/drugs). Nivolumab interacting with the N-terminal loop of PD-1 (6) failed to show benefits in a phase 3 clinical trial (CheckMate 026) for the treatment of stage IV NSCLC patients with PD-L1 tumor expression ≥1% (10). In contrast, pembrolizumab interacting with the PD-L1-binding IgV domain of PD-1 (5) successfully showed benefits in a phase 3 clinical trial (KEYNOTE-024) for the treatment of advanced NSCLC patients with PD-L1 tumor expression ≥50% (11). Although nivolumab and pembrolizumab exert anti-tumor effects through a common mechanism of the PD-1 signaling blockade, there are some functional divergences between nivolumab and pembrolizumab (Figure 1).
Lung cancers are classified into small cell lung cancer (SCLC), lung adenocarcinoma, lung squamous cell carcinoma (SCC) and other subtypes. Lung cancers other than SCLC have been traditionally categorized as NSCLC because of their relative unresponsiveness to combination chemotherapy; however, recent progress in genomic sequencing technology revealed subtype-specific genetic alterations in lung cancers. Gene amplification of the FGFR1 gene preferentially occurs in lung SCC and SCLC, whereas driver mutations in the ALK, EGFR, HER2, NTRK1, RET and ROS1 genes preferentially occur in lung adenocarcinoma (18-20). The standard therapy for patients with advanced lung SCC is platinum-based combination chemotherapy because FGFR inhibitors are not yet approved for the treatment of cancer patients, whereas initial therapy for advanced lung adenocarcinoma patients with the driver mutations mentioned above are receptor tyrosine kinase (RTK)-targeted therapies (20-22). Due to the distinct genomic landscapes of and therapeutic options for lung SCC and lung adenocarcinoma, NSCLC are further divided into squamous NSCLC and non-squamous NSCLC.
Pembrolizumab is a representative immuno-oncology drug for NSCLC patients (23-26). The phase 3 clinical trial KEYNOTE-024 demonstrated the superiority of pembrolizumab monotherapy over platinum-based standard chemotherapy as the first-line treatment for PD-L1-positive NSCLC patients without EGFR or ALK driver mutations (11). Both the pembrolizumab monotherapy group and platinum-based chemotherapy group included approximately 20% squamous NSCLC and 80% non-squamous NSCLC patients and showed almost similar safety profiles. The median progression-free survival (PFS) of the pembrolizumab monotherapy group and chemotherapy group was 10.3 and 6.0 months, respectively (hazard ratio, 0.50; 95% confidence interval, 0.37–0.68; P<0.001). In addition, the overall response rate (ORR) of the pembrolizumab monotherapy group and chemotherapy group was 44.8% and 27.8%, respectively. Pembrolizumab established its role as the first-line therapy for NSCLC patients based on the results of the KEYNOTE-024 clinical trial (11). However because the benefits of pembrolizumab monotherapy are limited to approximately 15% of NSCLC patients irrespective of the PD-L1 status, it was conceived that a combination strategy using pembrolizumab and platinum-based standard chemotherapy might enhance the benefits of pembrolizumab treatment for NSCLC patients.
Recently, Dr. Paz-Ares and colleagues reported the promising results of a phase 3 clinical trial of combination immune-oncology therapy with pembrolizumab and standard chemotherapy for the first-line treatment of patients with metastatic squamous NSCLC (KEYNOTE-407, NCT02775435) (27). The incidence of grade ≥3 adverse events for the chemotherapy (carboplatin and paclitaxel/nab-paclitaxel) plus pembrolizumab group and chemotherapy alone group was 69.8% and 68.2%, respectively, whereas the ORR of chemotherapy plus pembrolizumab group and chemotherapy alone group were 58.4% and 35.0%, respectively (P=0.0004), and the median PFS of the chemotherapy plus pembrolizumab group and chemotherapy alone group was 6.4 months and 4.8 months, respectively (hazard ratio, 0.56; 95% confidence interval, 0.45–0.70; P<0.0001). In contrast, Dr. Gandhi and colleagues reported the promising results of a phase 3 clinical trial of combination immune-oncology therapy with pembrolizumab and standard chemotherapy for the first-line treatment of patients with metastatic non-squamous NSCLC without EGFR or ALK driver mutations (KEYNOTE-189, NCT02578680) (28). The incidence of grade ≥3 adverse events for the chemotherapy (pemetrexed and a platinum-based drug) plus pembrolizumab group and chemotherapy alone group was 67.2% and 65.8%, respectively, whereas the median PFS of the chemotherapy plus pembrolizumab group and chemotherapy alone group was 8.8 and 4.9 months, respectively (hazard ratio, 0.52; 95% confidence interval, 0.43–0.64; P<0.001). Together, these facts clearly indicate that combination with standard chemotherapy significantly enhances the benefits of pembrolizumab for squamous as well as non-squamous NSCLC patients. Synergy between pembrolizumab and chemotherapy is a hot issue in the field of clinical oncology (29).
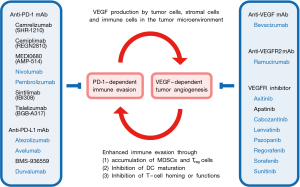
Combination with therapeutics targeting the tumor microenvironment is another strategy to enhance the benefits of immune checkpoint blockers for cancer patients (Figure 2). Aberrant VEGF signaling in the tumor microenvironment leads to a leaky and hypoxic condition that promotes the survival of cancer stem cells, the epithelial-to-mesenchymal transition of tumor cells, and immune evasion through the recruitment of myeloid-derived suppressor cells (MDSCs) and regulatory T (Treg) cells and the functional suppression of CD8+ T cells and natural killer (NK) cells (30-33). Anti-VEGF mAb (bevacizumab), anti-VEGFR2 mAb (ramucirumab) and VEGFR inhibitors (axitinib, cabozantinib, lenvatinib, pazopanib, regorafenib, sorafenib and sunitinib) are VEGF signaling targeted therapeutics (34-37) that are approved by the US FDA for the treatment of cancer patients, whereas apatinib (38) is a small-molecule VEGFR2 inhibitor that is approved by the Chinese FDA for the treatment of gastric cancer patients. Synergistic effects of immune checkpoint blockers and VEGF signaling blockers (Figure 2) have been investigated in the following clinical trials for the treatment of cancer patients: pembrolizumab plus apatinib (NCT03407976); pembrolizumab plus axitinib (NCT02853331); pembrolizumab plus bevacizumab (NCT02681549); pembrolizumab plus cabozantinib (NCT03149822); pembrolizumab plus lenvatinib (NCT02501096); pembrolizumab plus pazopanib (NCT02014636); pembrolizumab plus ramucirumab (NCT02443324); pembrolizumab plus regorafenib (NCT03347292); pembrolizumab plus sorafenib (NCT03211416) and pembrolizumab plus sunitinib (NCT03463460). Among these clinical trials, pembrolizumab-based combination therapies with bevacizumab or lenvatinib are in progress for the treatment of NSCLC patients.
The exploration and establishment of predictive biomarkers for patient selection are also necessary to enhance the benefits of immuno-oncology therapies. The immunohistochemistry-based detection of PD-L1 protein upregulation on tumor cells or tumor-associated macrophages (11) (Reck et al., 2016), reverse phase protein array (RPPA)-based detection of exosomal PD-L1 protein upregulation after immuno-oncology therapy (39), CD14+CD16-HLA-DRhigh monocytes in peripheral blood mononuclear cells (40), mismatch-repair deficiency (41) and higher tumor mutational burden (42) are biomarkers to predict responders to PD-1 signaling blockade therapy. In contrast, loss-of-function alterations in the JAK1/2 and beta-2-microglobulin (B2M) genes (43) and loss of neoantigens (44) are detected in cases with resistance to the immune checkpoint blockers. Among these predictive biomarkers to stratify or monitor cancer patients, liquid biopsy tests detecting exosomal PD-L1 protein and CD14+CD16−HLA-DRhigh monocytes are both promising technologies that might drastically improve the benefit-cost ratio of PD-1 blockade therapy.
In conclusion, the combinatorial optimization of immune checkpoint blockers, VEGF signaling blockers and cytotoxic chemotherapies as well as the development of biomarkers for the positive and negative selection of patients are necessary for the beneficial maximization of immuno-oncology drugs for the treatment of NSCLC patients and other types of cancer patients.
Acknowledgements
This work was supported in part by a grant-in-aid from M Katoh’s Fund for the Knowledgebase Project.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017;168:707-23. [Crossref] [PubMed]
- Nishino M, Ramaiya NH, Hatabu H, et al. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol 2017;14:655-68. [Crossref] [PubMed]
- Katoh M. Combination immuno-oncology therapy with immune checkpoint blockers targeting PD-L1, PD-1 or CTLA4 and epigenetic drugs targeting MYC and immune evasion for precision medicine. J Thorac Dis 2018;10:1294-9. [Crossref] [PubMed]
- Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med 2016;375:1767-78. [Crossref] [PubMed]
- Na Z, Yeo SP, Bharath SR, et al. Structural basis for blocking PD-1-mediated immune suppression by therapeutic antibody pembrolizumab. Cell Res 2017;27:147-50. [Crossref] [PubMed]
- Tan S, Zhang H, Chai Y, et al. An unexpected N-terminal loop in PD-1 dominates binding by nivolumab. Nat Commun 2017;8:14369. [Crossref] [PubMed]
- Huang J, Xu B, Mo H, et al. Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res 2018;24:1296-304. [Crossref] [PubMed]
- Migden MR, Rischin D, Schmults CD, et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med 2018;379:341-51. [Crossref] [PubMed]
- Hamid O, Chow LQ, Sanborn RE, et al. Combination of MEDI0680, an anti-PD-1 antibody, with durvalumab, an anti-PD-L1 antibody: A phase 1, open-label study in advanced malignancies. Ann Oncol 2016;27:1050PD.
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Yue C, Jiang Y, Li P, et al. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. Oncoimmunology 2018;7. [Crossref] [PubMed]
- Zhang T, Song X, Xu L, et al. The binding of an anti-PD-1 antibody to FcγRI has a profound impact on its biological functions. Cancer Immunol Immunother 2018;67:1079-90. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 2017;18:599-610. [Crossref] [PubMed]
- Gay CL, Bosch RJ, Ritz J, et al. Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 Infected Participants on Suppressive Antiretroviral Therapy. J Infect Dis 2017;215:1725-33. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Perez-Moreno P, Brambilla E, Thomas R, et al. Squamous cell carcinoma of the lung: molecular subtypes and therapeutic opportunities. Clin Cancer Res 2012;18:2443-51. [Crossref] [PubMed]
- Tan WL, Jain A, Takano A, et al. Novel therapeutic targets on the horizon for lung cancer. Lancet Oncol 2016;17:e347-62. [Crossref] [PubMed]
- Hirsch FR, Suda K, Wiens J, et al. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 2016;388:1012-24. [Crossref] [PubMed]
- Gandara DR, Hammerman PS, Sos ML, et al. Squamous cell lung cancer: from tumor genomics to cancer therapeutics. Clin Cancer Res 2015;21:2236-43. [Crossref] [PubMed]
- Katoh M. Therapeutics targeting FGF signaling network in human diseases. Trends Pharmacol Sci. 2016;37:1081-96. [Crossref] [PubMed]
- Remon J, Besse B, Soria JC. Successes and failures: what did we learn from recent first-line treatment immunotherapy trials in non-small cell lung cancer? BMC Med 2017;15:55. [Crossref] [PubMed]
- Ernani V, Ganti AK. Immunotherapy in treatment naïve advanced non-small cell lung cancer. J Thorac Dis 2018;10:S412-21. [Crossref] [PubMed]
- Peters S, Kerr KM, Stahel R. PD-1 blockade in advanced NSCLC: A focus on pembrolizumab. Cancer Treat Rev 2018;62:39-49. [Crossref] [PubMed]
- Brahmer JR, Govindan R, Anders RA, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J Immunother Cancer 2018;6:75. [Crossref] [PubMed]
- Paz-Ares LG, Luft A, Tafreshi A, et al. Phase 3 study of carboplatin-paclitaxel/nab-paclitaxel (Chemo) with or without pembrolizumab (Pembro) for patients (Pts) with metastatic squamous (Sq) non-small cell lung cancer (NSCLC). J Clin Oncol 2018;36:abstr 105.
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Sidaway P. Pembrolizumab synergizes with chemotherapy. Nat Rev Clin Oncol 2018;15:402-3. [Crossref] [PubMed]
- Katoh M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis. Int J Mol Med 2016;38:3-15. [Crossref] [PubMed]
- Manegold C, Dingemans AC, Gray JE, et al. The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol 2017;12:194-207. [Crossref] [PubMed]
- Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol 2018;15:310-24. [Crossref] [PubMed]
- Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018;15:325-40. [Crossref] [PubMed]
- Jayson GC, Kerbel R, Ellis LM, et al. Antiangiogenic therapy in oncology: current status and future directions. Lancet 2016;388:518-29. [Crossref] [PubMed]
- Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017;20:185-204. [Crossref] [PubMed]
- Wang J, Chen J, Guo Y, et al. Strategies targeting angiogenesis in advanced non-small cell lung cancer. Oncotarget 2017;8:53854-72. [PubMed]
- Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol 2018;19:405-15. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018;560:382-6. [Crossref] [PubMed]
- Krieg C, Nowicka M, Guglietta S, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med 2018;24:144-53. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-Programmed Cell Death (PD)-1 and anti-Programmed Death-Ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol 2018;36:633-41. [Crossref] [PubMed]
- Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 2016;375:819-29. [Crossref] [PubMed]
- Anagnostou V, Smith KN, Forde PM, et al. Evolution of neoantigen landscape during Iimmune checkpoint bockade in non-small cell lung cancer. Cancer Discov 2017;7:264-76. [Crossref] [PubMed]


