Lobar versus sub-lobar surgery for pulmonary typical carcinoid, a population-based analysis
Introduction
Pulmonary carcinoids (PCs) comprise 1−2% of all lung cancers with an age-adjusted incidence rate of 0.2−2/100,000 population/year (1). PCs are divided into TC and atypical carcinoid (AC) according to the WHO classification which was modified in 1999 (2). TC is more common than AC and represents more than 80% of PCs (3). Complete surgical resection is the treatment of choice for resectable localized and loco-regional PCs. Five-year survival rate for TC and AC range between 87−97% and 56−77%, respectively (4).
The optimal surgery for resectable PC is still an issue of controversy. Options include SL-R (wedge or segmental) versus more extensive surgery such as lobectomy or pneumonectomy without having randomized trials to favor a specific approach. The evidence supporting resection of PCs is largely based on cancer recurrence and survival outcomes reported in case series and population-based studies. Previous studies using SEER data (5-7), showed that sub-lobar resection (SL-R) of PCs was not inferior to lobar resection (L-R). However, these studies differed in their methodology; the time periods covered, and were limited by the lack of adjustment for the use of adjuvant treatments and comorbid conditions. Other studies showed that SL-R is appropriate for pulmonary TC, but a more extensive surgery, with lymph node dissection, might be needed for AC (4,8,9). Cardillo et al. (10) suggested that lymph node status was more important than the exact histology and concluded in favor of anatomic resection and radical mediastinal lymphadenectomy for both TC and AC.
In this study, we addressed the limitations of SEER data by including SEER-Medicare data, which allow us to control for the use of adjuvant treatments and comorbid conditions when comparing survival outcomes by type of surgery among TC patients. Our analyses used data collected after 1999 when WHO published a new classification of PCs (2).
Methods
Subject selection
This study was approved by the University of Iowa IRB (IRB #201304798) and subject to a data use agreement with NCI. The study primarily used data from the SEER program with a supplemental, sensitivity analysis conducted using SEER-Medicare data. The SEER program is a collection of population-based cancer registries, covering 28% percent of the U.S. population (11). SEER data contains information on patient and tumor characteristics, initial course of local therapy, and survival. Linked to Medicare administrative claims, SEER-Medicare data provide additional information regarding utilization of covered health care services for Medicare beneficiaries.
The cohort of this study extracted from SEER data consisted of patients: (I) newly diagnosed with pathologically confirmed cases of pulmonary TC (ICD-O-3 code 8240 site code C34) and non-small cell lung cancer (NSCLC) from 2000−2012, (II) aged 66 or older at the time of diagnosis (age restriction to allow including SEER-Medicare comparable patients as mentioned below), (III) with localized or regional disease per SEER Historical Stage A. Patients were excluded if (I) diagnosis was made at autopsy or death (N=175), (II) died within a month of cancer diagnosis (N=4,368), or (III) the surgery type was pneumonectomy (N=2,026) or unknown (N=562). A comparable study cohort was identified from the SEER-Medicare data, with diagnoses from 2000−2007, to control for receipt of CTX and to assess comorbidity status. Survival analyses using SEER data were restricted to 2000-2011 diagnoses to allow for sufficient follow up.
Measures
Patient demographics included patient age, sex and race. Age at diagnosis was categorized into less or equal to 75 and over 75. Race was grouped as white and non-white. Patient-specific socio-demographic information is not included in SEER. However, SEER provides the Health Service Area (HSA) associated with a patient’s residence. These HSAs were mapped to counties [five-digit Federal Information Processing Standard (FIPS) codes] to create rurality variables for patients, and classified as large metropolitan areas or not. The tumor stage was categorized into localized and regional disease according to SEER Historic Stage A. AJCC TNM staging system was not used as data were missing for many patients. Lung surgeries were categorized into L-R, SL-R or NS using the “site-specific surgery of primary site” variable (surgprim). SL-R included wedge resection (SEER surgery code 21), segmental resection (code 22) and “others” (codes 20, 23−25). As there was no statistically significant difference in relative survival between wedge and segmental resection (refer to the results section), the SL-R category was created by combining wedge and segmental resections, and “others”. Receipt of radiotherapy (XRT) was also identified. For the L-R and SL-R groups, adjuvant XRT was defined as radiation within 6 months of surgery. For the NS group, we included the first XRT delivered within a year of the diagnosis of pulmonary TC.
From SEER-Medicare data, comorbidity was determined from inpatient and outpatient Medicare claims for the year prior to lung cancer diagnosis using a modified Charlson comorbidity index scale (12). Chemotherapy (CTX) claims were identified and a patient was considered to have received CTX if the first claim was observed within six-months before or after surgery. For the NS group, CTX was included from the first claims within 8 months after diagnosis. Of note, somatostatin analogues were not treated as CTX and were not included in the CTX variable.
Statistical analyses
Chi-squared tests were conducted to compare patient and tumor characteristics by type of surgery. Fisher’s exact tests were conducted if cell sizes were small (the expected cell counts were less than 5). Survival was estimated by the Kaplan-Meier (KM) method and log-rank tests were conducted to assess differences in overall survival. Five-year relative survival (observed divided by expected survival) and cause-specific death were estimated using SEER*Stat, v8.3.2 (Information Management Services, Incorporated; Calverton, MD, USA). A multivariate Cox proportional hazards model using the SEER-Medicare linked database sample was fitted to examine the effect of surgery and its type on 2-year overall survival, controlling for age, sex, race, historical stage, receipt of adjuvant CTX and XRT, chronic obstructive pulmonary disease (COPD), and other comorbidity. Analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, North Carolina, USA). All tests were two-sided and statistical significance defined at the 5% level. Per SEER-Medicare protocol, cell sizes less than 11 are to be suppressed in the reporting of any results. In this manuscript, non-suppressed tables include SEER data only (Tables 1 and 2).
Full table

Full table
Results
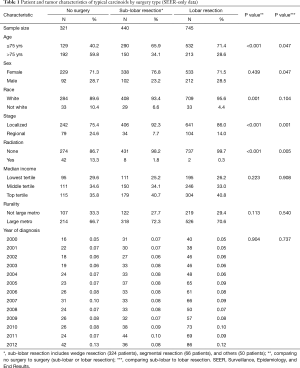
Our final SEER cohort included 1,506 patients with pulmonary TC. Of these, 745 (49%) received L-R, 440 (29%) received SL-R (wedge resection N=324, segmental resection N=66, and others N=50), and 321 (21%) received NS (Table 1). Age, race, stage, and receipt of XRT differed for those who did not receive surgery compared to those who did (L-R or SL-R). All these factors, except race, were also significant when comparing L-R to SL-R, although differences by surgery type were less pronounced. Of those who received surgery, L-R was associated with younger age, men, and being diagnosed with regional disease.
The impact of surgery, and surgery type, on overall survival for pulmonary TC and NSCLC were assessed with KM curves (Figure 1). Overall survival for pulmonary TCs and NSCLC was lower for those who did not receive surgery than those who did, with both P<0.001. In contrast, there was no difference in survival by type of surgery (L-R vs. SL-R) for either localized (P=0.209) or regional (P=0.364) pulmonary TC. In NSCLC, patients with SL-R had worse survival than patients with L-R (P<0.001 for both localized and regional disease). Regarding the effect of surgery versus no surgery (NS) on survival, the same results were observed when estimated 3- and 5-year overall survival was calculated using a propensity score matched model (nearest neighbor, one-to-one matching). The conclusions were the same (surgery was found to be associated with better survival, but not extent of surgery). Given that this was an ad hoc analysis and it did not alter any of the conclusions, we chose not to include the propensity score model results in the manuscript.

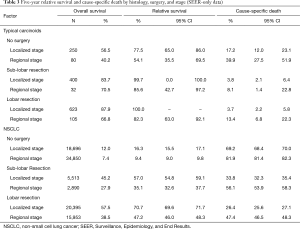
Five-year relative and cause-specific survival were significantly higher for both pulmonary TC and NSCLC if surgery was performed (Table 3). Five-year relative survival for patients with localized TCs who received any surgery was nearly 100%. There was no statistically significant difference in relative survival for TCs with localized disease (83.7% vs. 87.9%, P=1.000) or regional disease (70.5% vs. 66.8%, P=0.828) by surgery type. Relative survival was similar for wedge resection or segmental resection when compared to L-R (Table 2). In contrast, relative survival for NSCLC patients who received L-R was better than for those who received SL-R (P<0.001 for both localized and regional disease). For pulmonary TC, Cause-specific death for patients who received SL-R was relatively low for localized and regional stage (3.8% and 8.1%, respectively), along with those who received L-R (3.7% and 13.4%, respectively). In contrast, the majority of deaths for NSCLC were cause-specific, ranging from 56.1% for regional disease that received SL-R to 26.4% for localized disease that received L-R.
Full table
Our SEER-Medicare sample comprised of 512 patients. Patients who did not receive surgery were older (P<0.001), had greater comorbidity (P<0.001) and regional disease at diagnosis (P<0.001) compared to those who underwent surgery. Receipt of CTX (P≤0.001) and XRT (P<0.0001) was also more common in NS patients. Proportion of patients with COPD in surgery vs. NS group was similar (P=0.065). Across the two surgery groups, distribution of various variables was similar except for the patients who had lobectomy were more likely to receive adjuvant CTX (P=0.037).
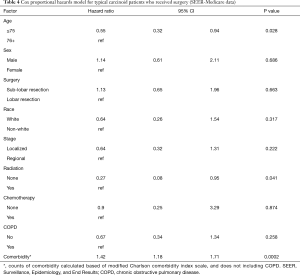
A multivariate Cox proportional hazards model was fitted for the pulmonary TC SEER-Medicare sample to assess the effect of surgery (none vs. L-R or SL-R) on overall survival, controlling for other covariates. In this model, younger age (HR 0.5; CI: 0.34−0.74), localized stage (HR 0.62; CI: 0.39−0.98), and not performing XRT (HR 0.43; CI: 0.22−0.86) were associated with better survival, while male gender (HR 1.65; CI: 1.11−2.47), NS (HR 3.36; CI: 2.28−4.96), and greater number of comorbidity (HR 1.19; CI: 1.04−1.34) were associated with increased risk of death.
Another multivariate Cox proportional hazards model was fitted using the resected pulmonary TC SEER-Medicare sample, to assess differences in survival by surgery type (excluding the patients who had NS), controlling for additional covariates such as receipt of CTX, COPD, and other comorbidity (Table 4). Younger age and less comorbidity were associated with better survival, and adjuvant XRT with poorer survival. There was no difference in survival between those who received L-R versus SL-R, after controlling for all other covariates, including stage.
Full table
Discussion
In this study, among elderly patients with pulmonary TC, there was no significant difference in survival between patients who received L-R or SL-R after adjusting for cancer stage (localized vs. regional), patient’s comorbidities and the use of adjuvant CTX or XRT. Wedge resection (74%) was the most common type of surgery in the SL-R group and the survival outcome of wedge resection was comparable to those of L-R. However, NS was associated with significant increase in pulmonary TC cause-specific death compared to L-R or SL-R.
Since there are no randomized-controlled trials to support a preferred type of surgical resection for pulmonary TC, principles of NSCLC surgery became the de facto approach. Therefore, we compared survival for both of these diseases according to the type of surgery performed. Showing worse survival for NSCLC patients who underwent SL-R compared to LR was consistent with the literature and gives validity to the dataset we used. The same applies for showing worse outcomes for NSCLC compared to TC. Five-year overall survival was significantly inferior for patients with NSCLC who underwent SL-R but this did not hold true for pulmonary TC when compared to L-R. This probably reflects the aggressive nature of NSCLC compared to pulmonary TC where anatomical resection of the cancer with sampling of hilar and mediastinal lymph nodes is of major importance. Randomized trials have already shown the superiority of lobectomy over limited resection in NSCLC (13), but this is unknown for pulmonary TC. It is also possible that 5-year follow up duration might not be enough to detect a small difference in survival conferred by larger resection for pulmonary TC; however such benefit should not be presumed especially for elderly patients. We understand that SL-R is not always feasible for patients with TC given the tumor location or nodal involvement, but our study and other population-based studies (4,5,7) are among the largest in terms of sample size on this issue, and all favor limited resection if performing L-R is unsafe. It can be actually argued that SL-R might be the treatment of choice for peripheral TC, however, a randomized trial or prospective registry could provide further guidance as to whether SL-R can replace L-R in pulmonary TC. We also attempted to investigate outcomes of patients with AC in a similar fashion; due to the small number of cases in the data-set, we could not draw reliable conclusions (data not shown).
Findings of our study are consistent with previously published reports (4,5,7), but we did select a different patient population and adjusted outcomes for additional confounding variables. Travis et al. re-defined pulmonary TC in 1998 as pulmonary neuroendocrine (NE) tumors with mitotic count of less than 2 per 2 mm2 (10 HPF) with no evidence of coagulative necrosis (14) as opposed to the original Argoni classification, which considered NE tumors with less than 5 mitosis per 2 mm2 as TC (2,15). Travis classification was adopted by WHO in 1999 and it clearly separated TC from AC as a prognostically different subset of NE tumors. Therefore, prior studies which included TC patients diagnosed before 1999 (5,6), many of those may not be categorized as TC per the current classification. Raz et al. (6) also excluded patients who either had nodal disease or underwent XRT, and did not include data about CTX or comorbid conditions, whereas Yendamuri et al. (5), evaluated patients who underwent pneumonectomy or lobectomy together in the same group of ‘L-R’. Though pneumonectomy is potentially a curative surgery for patients with centrally located pulmonary TC, the morbidity and mortality of the procedure is higher than less extensive resections. Whether pneumonectomy should be offered as a treatment option if a “lesser surgery” is not feasible is unknown. To proceed with pneumonectomy will likely be a decision driven by factors such as patient’s age, life expectancy, comorbid conditions, and pulmonary functions. Therefore, we compared L-R vs. SL-R and excluded patients who received pneumonectomy. Fox et al. (7) also examined SEER database but they reported composite outcomes for pulmonary TC and AC. Additionally, all of these authors could not assess the impact of factors like COPD, other comorbidities and receipt of adjuvant CTX on patients’ outcomes.
The role of adjuvant CTX and/or XRT has not been assessed for pulmonary TC in randomized studies. However, some of the patients in our sample had received adjuvant therapies. Through SEER-Medicare linked data-set, we were able to assess the role of adjuvant CTX. Though number of patients who received adjuvant CTX was small, we did not see survival advantage from it. Another group of investigators actually found a trend towards harm from adjuvant CTX in this patient population (16). Similarly, adjuvant XRT was also associated with detrimental effect on survival in the SEER-Medicare cohort of our study. Notably, adjuvant therapy is often advocated for patients who either have higher stage or residual disease which could be the case in the LR group in our study as they were more likely to receive adjuvant CTX compared to the SL-R group (14% vs. 7.7%). This intrinsic bias might also explain the negative effect noticed with the adjuvant strategy.
When surgery cannot be performed, XRT and/or bronchoscopy guided ablative procedures (laser, cryosurgery, or thermal ablation) are sometimes employed. We noticed, 22% of the patients in our study did not receive surgery and this was associated with worse overall survival. The majority of these patients were older than 75 years (67%) and had localized disease (74%) with greater comorbidity. There was no significant difference in the COPD rates between the NS and surgery groups (P=0.065) which is likely a reflection of poor association of TC with smoking. As anticipated, a significant proportion of the patients in the NS group received XRT. Besides XRT, we could not reliably assess if these NS patients received other treatment modalities such as bronchoscopic guided ablative procedures or other therapies. Unexpectedly, XRT did not provide survival advantage over “NS”; instead it was associated with inferior survival. This was independent of patients’ age, sex, race, cancer stage and comorbid conditions. It is important to interpret this with caution, as the patients in the NS group might have received other alternative therapies, and impact of those therapies could not be assessed due to the retrospective nature of our study.
This study has several limitations that are inherent to any retrospective analyses of SEER database. Some of those include lack of information about tumor location (central vs. peripheral), selection bias for the treatment assigned, type of resection (R0, R1 or R2), incomplete staging for patients who did not receive surgery, inability to obtain TNM clinical and pathological staging, lack of data about recurrence, and therapies received after recurrence such as the exact chemotherapies delivered or radiation schedules. Randomized trials or prospective registries could provide more definitive answers about the optimal surgical management of pulmonary TC; however such efforts will be challenging to organize for this rare disease.
Conclusions
In the absence of prospective evidence, our data support SL-R, over NS as an acceptable intervention in the elderly patients with pulmonary TC if L-R is unfeasible. We complement the findings of other investigators on the optimal surgery for pulmonary TC by adjusting patient’s outcomes for comorbidities and receipt of adjuvant therapies. Prospective trials are needed to assess the role of XRT for inoperable pulmonary TC as this was associated with worse survival in our study.
Acknowledgements
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Funding: This work was supported in part by the Holden Comprehensive Cancer Center’s Population Science Pilot Award through the National Cancer Institute of the National Institutes of Health under Award Number P30CA086862. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the University of Iowa IRB (IRB #201304798) and subject to a data use agreement with NCI.
References
- Caplin ME, Baudin E, Ferolla P, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol 2015;26:1604-20. [Crossref] [PubMed]
- Travis W CT, Corrin B et al. Histological Typing of Lung and Pleural Tumors. Berlin H, New York: Springer-Verlag, 1999.
- Fink G, Krelbaum T, Yellin A, et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001;119:1647-51. [Crossref] [PubMed]
- Rea F, Rizzardi G, Zuin A, et al. Outcome and surgical strategy in bronchial carcinoid tumors: single institution experience with 252 patients. Eur J Cardiothorac Surg 2007;31:186-91. [Crossref] [PubMed]
- Yendamuri S, Gold D, Jayaprakash V, et al. Is sublobar resection sufficient for carcinoid tumors? Ann Thorac Surg 2011;92:1774-8; discussion 1778-9.
- Raz DJ, Nelson RA, Grannis FW, et al. Natural history of typical pulmonary carcinoid tumors: a comparison of nonsurgical and surgical treatment. Chest 2015;147:1111-7. [Crossref] [PubMed]
- Fox M, Van Berkel V, Bousamra M 2nd, et al. Surgical management of pulmonary carcinoid tumors: sublobar resection versus lobectomy. Am J Surg 2013;205:200-8. [Crossref] [PubMed]
- Mezzetti M, Raveglia F, Panigalli T, et al. Assessment of outcomes in typical and atypical carcinoids according to latest WHO classification. Ann Thorac Surg 2003;76:1838-42. [Crossref] [PubMed]
- Ferguson MK, Landreneau RJ, Hazelrigg SR, et al. Long-term outcome after resection for bronchial carcinoid tumors. Eur J Cardiothorac Surg 2000;18:156-61. [Crossref] [PubMed]
- Cardillo G, Sera F, Di Martino M, et al. Bronchial carcinoid tumors: nodal status and long-term survival after resection. Ann Thorac Surg 2004;77:1781-5. [Crossref] [PubMed]
- Surveillance Epidemiology and End Results (SEER) Program. National Cancer Institute. Available online: www.seer.cancer.gov. Accessed May 23 2016.
- Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258-67. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Travis WD, Rush W, Flieder DB, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol 1998;22:934-44. [Crossref] [PubMed]
- Arrigoni MG, Woolner LB, Bernatz PE. Atypical carcinoid tumors of the lung. J Thorac Cardiovasc Surg 1972;64:413-21. [PubMed]
- Nussbaum DP, Speicher PJ, Gulack BC, et al. Defining the role of adjuvant chemotherapy after lobectomy for typical bronchopulmonary carcinoid tumors. Ann Thorac Surg 2015;99:428-34. [Crossref] [PubMed]


