Mediastinal lymphadenopathy reflecting disease activity in an infant with chronic pneumonitis of infancy associated with surfactant protein C mutation: a case report and literature review
Introduction
Chronic pneumonitis of infancy (CPI) is a rare interstitial lung disease (ILD) that can be fatal in some cases. It occurs in infants who initially appear well and then develop respiratory symptoms with hypoxemia and diffuse interstitial infiltrates (1). Recently, the development of molecular genetic techniques has been rapidly increasing the understanding of the cause of CPI, and mutations in related genes encoding proteins such as surfactant protein (SP)-B, SP-C and ATP-binding cassette protein family A3 (ABCA3) have been identified as a representative cause (2).
Here, we report a 10-month-old girl who presented with hypoxemia and diagnosed as CPI with mediastinal lymphadenopathy on computed tomography (CT). In many literatures, the findings in CPI have been described only focusing on the lungs, and mediastinal lymphadenopathy has never been reported in patients with CPI. To the best of our knowledge, this is the first report to describe a case of CPI with mediastinal lymphadenopathy correlating to disease progression.
Case presentation
A 10-month-old previously healthy girl presented with a fever, cough, and cyanosis that developed within 2 days. She did not have any underlying disease such as a congenital heart defect and showed normal growth. Her mother was healthy, but her father had four surgeries for recurrent pneumothorax with unknown causes. Her parents noted mild cyanosis on her lips and her fingers but they could not remember when it first developed. Upon admission, the patient’s initial oxygen saturation at room air was 55–65% by pulse oximetry with mild respiratory distress and her initial oxygen partial pressure was 30.3 mmHg based on an arterial blood gas analysis. Oxygen was immediately given by a mask with a 5 L/min flow, the patient’s oxygen saturation recovered to 98% and her arterial oxygen partial pressure was increased to 68.8 mmHg.
Physical examinations revealed coarse breathing sounds on both lung fields with mild subcostal retractions without crackles or wheezing. Initial chest radiography showed non-specific diffuse bilateral reticular infiltrations.
Laboratory testing revealed mild leukocytosis (14,400/mm3) with 77.4% neutrophils, 2.0 mg/dL of C-reactive protein and 1,402 IU/L (normal range in 1 month to 5 years: 150–360 IU/L at 37 °C) of lactate dehydrogenase (LDH). An initial test for immunoglobulin M against Mycoplasma and cultures from blood/urogenital were negative; the only positive result was rhinovirus by respiratory virus PCR. Neither sweat test nor cystic fibrosis (CF) genetic test was performed. The levels of immunoglobulin G, A, M, E were checked for immunologic screening which was normal. Other immunologic work-ups were not performed because of their economical status.
Transthoracic echocardiography revealed a structurally and functionally normal heart. Chest CT with angiography revealed bilateral ground-glass opacity (GGO) in the lung parenchyma without any cystic lesions, mild dilatation of the main pulmonary artery, enlarged mediastinal lymph nodes (LNs), and there was no evidence of an arteriovenous fistula (Figure 1).
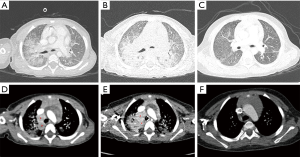
The CT findings were considered to be highly probable for bacterial pneumonia and empirical antibiotic treatment was started. She did not improve despite two weeks of empirical antibiotic therapy and therefore performed bronchoscopy to assess endotracheal lesions or congenital airway anomalies which resulted as normal. Bronchoalveolar lavage (BAL) fluid samples were obtained and cell differential counts were as following: 3,700/mm3 of RBC, 163/mm3 of WBC (neutrophil, 47%, lymphocyte, 32% and monocyte 19%) and also numerous alveolar macrophages were noted. Studies for tuberculosis PCR, cytospin, and culture with BAL fluid were all negative. As the symptom of patients aggravates, the chest CT with contrast was followed up for assessment of treatment response which revealed aggravation of multiple enlarged mediastinal LNs in both paratracheal, hilar, and interlobar areas with increased density of diffuse GGOs in both lung fields. With suspicion of a lymphoproliferative disease such as lymphoma or Castleman’s disease, the CT scan of the neck and abdomen was also performed which was normal and an open biopsy of the mediastinal LNs was performed which also resulted in only reactive lymphadenitis with negative Langerin stain (Figure 1).
After ruling out our differential diagnosis of congenital heart defect, arteriovenous fistula, endobronchial lesions and possible concomitant malignancy, the patient was diagnosed with childhood ILD and given intravenous methylprednisolone (MPD) (20 mg/kg for 3 days, monthly) and home oxygen (2 L/min via nasal prongs) therapy.
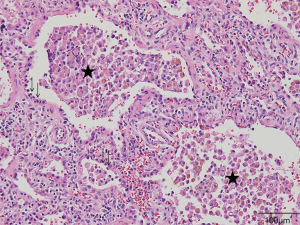
The patient underwent a planned open lung biopsy, 4 months after MPD therapy as the parents wanted to postpone the general anesthesia after the mediastinal LN biopsy. A histologic evaluation revealed diffuse interstitial thickening and lymphocyte infiltration and intra-alveolar macrophage accumulation with type II pneumocyte hyperplasia, compatible with CPI (Figure 2).
Genetic analysis
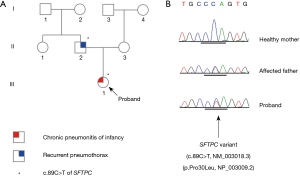
Whole-exome sequencing (WES) was performed in our proband using SureSelect Human All Exon V5 (Agilent Technologies, Santa Clara, CA, USA) on the Illumina NextSeq500 platform (Illumina Inc., San Diego, CA, USA). Variants were called using the Genome Analysis Toolkit (version 3.2-2) and prioritized through our bioinformatics workflow. Common variants (allele frequency, >0.01) were filtered out referring to known single nucleotide polymorphism (SNP) databases, including the Korean Reference Genome Database (http://152.99.75.168/KRGDB/menuPages/intro.jsp in Korean; 622 normal Korean controls). A missense mutation in the gene encoding surfactant pulmonary-associated protein C (SFTPC) was found (c.89C>T; p.Pro30Leu) under the autosomal dominant model. It was not identified in any SNP database. The candidate variant was verified in our proband based on Sanger sequencing; it was found in the patient’s affected father but not in her healthy mother (Figure 3).
After the ninth cycle of MPD pulse therapy, the patient’s oxygen demand decreased significantly and she was weaned off of home oxygen therapy. Although her symptoms improved, the patient’s radiologic findings changed over the year. Chest CT revealed centrilobular emphysema in both upper lobes, paraseptal emphysema in both upper lobes, and an aggravated superior segment in both lower lobes; however, her mediastinal LNs decreased significantly as her symptoms improved (Figure 1).
Discussion
In this present case, the infant girl presented with severe hypoxemia and mediastinal lymphadenopathy was found on CT. Although we could diagnose her as CPI associated with an SFTPC gene mutation based on an open lung biopsy and WES, mediastinal lymphadenopathy complicated the diagnosis. The mediastinal lymphadenopathy deteriorated during the empirical treatment and began to improve with anti-inflammatory treatment for CPI and finally regressed. This suggests that the disease progression and lymphadenopathy may have some relationship in the patients with CPI.
According to the Official American Thoracic Society Documents of 2013, our patient was classified with an SFTPC genetic mutation-CPI dominant histologic pattern (2). They recommended that children less than 2 years should be excluded the diseases like CF, congenital or acquired immunodeficiency, congenital heart disease, bronchopulmonary dysplasia, pulmonary infection, and primary ciliary dyskinesia before suspicion of childhood ILD. To rule out such underlying diseases, bronchoscopy with BAL and echocardiography are suggested. After that, childhood ILDs are diagnosed based mainly on family history, genetic testing and lung biopsy. If the evaluations are negative or symptoms persist despite treatment, high resolution CT (HRCT) is highly recommended (2). CF is one of the diseases that should be ruled out, but the tests were not performed in our patient in the first place, because it occurs very rarely in Asians compared to Caucasians (3).
In the present case, we performed HRCT to identify underlying lung lesions and incidentally detected multiple enlarged mediastinal LNs. Mediastinal lymphadenopathy, which complicated the diagnosis of ILD initially, was suggestive of a lymphoproliferative disease such as lymphoma. However, further evaluation could not identify any malignant lesions.
The mediastinal LN enlargement in patients with CPI or in a patient with childhood ILD has not been reported in a pediatric population. However, Attili et al. (4) reported the correlation between mediastinal LN enlargement and disease activity in adult patients with usual interstitial pneumonitis and non-specific interstitial pneumonitis. They showed that an increase in LN enlargement over time is correlated with the progression of fibrosis. Souza et al. (5) also reported the prevalence of mediastinal LN enlargement in adult patients with idiopathic interstitial pneumonia. They concluded that mediastinal LN enlargement is a common finding in adult patients with idiopathic interstitial pneumonia. For this present case, we could find correlation between mediastinal LN enlargement and disease activity, as well.
The pathogenesis of enlarged mediastinal LNs in patients with ILD can be considered an evoked inflammatory response (6). Surfactants are recycled by type II pneumocytes or catabolized by alveolar macrophages to maintain precise levels in a highly regulated system. The mutation of genes associated with surfactant production such as SFTPC gene result in the formation of misfolded proteins, and the alveolar macrophages cannot catabolize them properly and are continuously activated (7). LN enlargement is induced by cytokines released by activated alveolar macrophages. Excessive secretion of profibrotic and proinflammatory cytokines such as interleukin (IL)-4, IL-5, transforming growth factor-β and tumor necrosis factor-α are implicated in the pathogenesis of pulmonary fibrosis (8). On the other side, it is known as SP-A which binds to and removes several microbial pathogens by attaching to the lipopolysaccharide (LPS) domain (7). Glasser et al. (9) suggested that SP-C may also have a partial role in clearing pathogen and reducing inflammation. They injected LPS of gram negative bacteria into SFTPC knockout mice, and SP-C-deficient mice were found to have increased inflammatory response and increased inflammation with repeated exposure to LPS. In the present case, a missense mutation in SFTPC was identified (c.89C > T; p.Pro30Leu) under the autosomal dominant model. It was not found in any SNP database and may represent a novel mutation.
In addition, Jung et al. (10) showed that the greater the severity score of pulmonary fibrosis, the higher the number of enlarged LNs suggesting a correlation between fibrotic lung lesions and mediastinal LNs. Although further study is required to explore the correlation between mediastinal LN enlargement and CPI in terms of disease severity, our patient had initial aggravation of LN enlargement prior to the treatment, and after 9 months of MPD and oxygen therapy, the mediastinal LN enlargements improved.
The clinical courses of CPI in pediatric population vary widely and are summarized in Table 1. We found three patients in two case reports who survived and improved after appropriate diagnosis and supportive treatment for 50 days to 37 months (13,14). The reported treatment of CPI included hydroxychloroquine, steroids (MPD or dexamethasone), and antibiotics in survived patients. Those who expired tend to be younger (1-day-old and 33-day-old) than those who survived (2–4 months) (11-14).
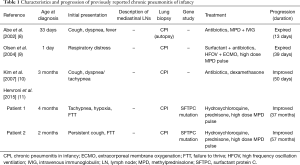
Full table
Our patient weaned off of oxygen and obtained symptomatic improvement after MPD and home oxygen therapy for 9 months. However, considering the progressive characteristics of the disease and her high life expectancy, we were concerned that there is no definitive treatment for CPI.
Among the available pharmacological therapies, immunosuppressive therapy (e.g., systemic corticosteroids and hydroxychloroquine) is most commonly used in children, but the effectiveness is unclear. Lung transplantation is an option for infants and children with severe, life-threatening childhood ILDs. Further development of treatment modalities, such as gene therapy is crucial in childhood ILDs, including CPI. We performed a genetic evaluation not just for the purpose of diagnosis, but in the hope of developing new treatment modalities such as gene therapy.
Conclusions
We diagnosed and managed an infant with CPI who had an SFTPC mutation, however, mediastinal LN enlargement was a confusing factor mimicking a lymphoproliferative disease such as lymphoma. Mediastinal lymphadenopathy is an unusual finding of ILD in children and it correlated to disease activity in our case. Mediastinal lymphadenopathy could be considered as a factor reflecting disease progression in patients with ILD. Further study is required to characterize the correlation of disease activity between mediastinal LN enlargement and ILD in pediatric populations.
Acknowledgements
Young-Eun Kim (Greencross Genome, Yongin, Republic of Korea) helped us with interpretation of whole exome sequencing of this patient.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Langston C, Fan LL. Diffuse interstitial lung disease in infants. Pediatr Pulmonol 2001.Suppl 23:74-6. [Crossref] [PubMed]
- Kurland G, Deterding RR, Hagood JS, et al. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med 2013;188:376-94. [Crossref] [PubMed]
- Ahn KM, Park HY, Lee JH, et al. Cystic fibrosis in Korean children: a case report identified by a quantitative pilocarpine lontophoresis sweat test and genetic analysis. J Korean Med Sci 2005;20:153-7. [Crossref] [PubMed]
- Attili AK, Kazerooni EA, Gross BH, et al. Thoracic lymph node enlargement in usual interstitial pneumonitis and nonspecific-interstitial pneumonitis: prevalence, correlation with disease activity and temporal evolution. J Thorac Imaging 2006;21:288-92. [Crossref] [PubMed]
- Souza CA, Muller NL, Lee KS, et al. Idiopathic interstitial pneumonias: prevalence of mediastinal lymph node enlargement in 206 patients. AJR Am J Roentgenol 2006;186:995-9. [Crossref] [PubMed]
- Warrick JH, Bhalla M, Schabel SI, et al. High resolution computed tomography in early scleroderma lung disease. J Rheumatol 1991;18:1520-8. [PubMed]
- Whitsett JA, Wert SE, Weaver TE. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu Rev Med 2010;61:105-19. [Crossref] [PubMed]
- Coker RK, Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur Respir J 1998;11:1218-21. [Crossref] [PubMed]
- Glasser SW, Maxfield MD, Ruetschilling TL, et al. Persistence of LPS-induced lung inflammation in surfactant protein-C-deficient mice. Am J Respir Cell Mol Biol 2013;49:845-54. [Crossref] [PubMed]
- Jung JI, Kim HH, Jung YJ, et al. Mediastinal lymphadenopathy in pulmonary fibrosis: correlation with disease severity. J Comput Assist Tomogr 2000;24:706-10. [Crossref] [PubMed]
- Abe K, Kamata N, Okazaki E, et al. Chronic pneumonitis of infancy. Eur Radiol 2002;12 Suppl 3:S155-7. [PubMed]
- Olsen EO, Sebire NJ, Jaffe A, et al. Chronic pneumonitis of infancy: high-resolution CT findings. Pediatr Radiol 2004;34:86-8. [Crossref] [PubMed]
- Kim JM, Kwon SY, Kim ES, et al. A good outcome for a case of chronic pneumonitis of infancy. Yonsei Med J 2007;48:865-7. [Crossref] [PubMed]
- Hevroni A, Goldman A, Springer C. Infant pulmonary function testing in chronic pneumonitis of infancy due to surfactant protein C mutation. Pediatr Pulmonol 2015;50:E17-23. [Crossref] [PubMed]