Managing complications II: conduit failure and conduit airway fistulas
Introduction
Although decidedly uncommon, conduit necrosis and conduit airway fistula are two of the most feared complications after esophagectomy. Both are associated with high mortality and may result in loss of the conduit and need for additional operations to reestablish continuity of the gastrointestinal tract. The treatment course of survivors generally includes prolonged ICU and hospital stay as well as multiple additional interventions and procedures.
Conduit failure
Multiple systems exist to classify anastomotic leak. One commonly used example is the system developed by Lerut, et al. shown in Table 1 (1). The most severe category of leak in this and other systems includes those due to necrosis of a portion of the gastric conduit. The incidence of gastric conduit necrosis is reported between 0.5-3.2% (2-4). Orringer and colleagues reviewed 1,085 patients who underwent transhiatal esophagectomy with gastric tube conduit with a reported incidence of graft ischemia of 2.6% (5). Ramage and colleagues reviewed 155 patients who underwent minimally invasive esophagectomy and found a similar 2.6% incidence of conduit necrosis (6).

Full table
Risk factors
Risk factors for gastric conduit necrosis can be divided into patient-related factors, technical factors and postoperative care. Anatomic factors such as the lack of a serosal layer on the esophagus and the longitudinal orientation of the muscular layer have been implicated in anastomotic leak in general but not specifically related to conduit necrosis (7). It is important to understand the blood supply of the conduit. The majority of the gastric conduit is supplied directly by the right gastroepiploic artery with the remainder of the conduit supplied by a network of microvascular submucosal collaterals (8). The anastomosis is necessarily created within the most ischemic portion of the conduit. Therefore it is important when mobilizing the conduit to not only preserve the macroscopic blood supply but also avoid trauma to the submucosal vascular plexus.
Patient-related factors include peptic ulcer disease, history of external beam irradiation, and severe malnutrition (9). Peptic ulcer disease can cause local inflammation in the area of the conduit which will be used for anastomosis. External beam radiation leads to localized fibrosis and decreased microvascular network in the stomach and may affect the vascularity of the conduit. Severe malnutrition is not uncommon in patients with esophageal cancer as most have a variable period of time during which they will attempt to adjust their diet and adapt to the progressive dysphagia prior to seeking diagnosis and treatment. Patients with severe malnutrition may benefit from feeding jejunostomy tube placement with a period of nutritional support prior to proceeding with esophagectomy. Interestingly, both diabetes and perioperative steroid use have not been shown to correlate with anastomotic breakdown despite their well-known association with poor wound healing (10,11). Although age is commonly implicated as a risk factor for morbidity and mortality in complex operations, it has not been shown to be associated with conduit necrosis or other anastomotic complications (12).
Technical factors include mobilization of the stomach, creation and placement of the conduit, and anastomotic technique. The stomach is the most commonly used conduit for benign and malignant disease for several reasons: it is relatively easy to mobilize, it only requires one bowel anastomosis, and it has a fairly consistent right gastroepiploic arterial supply. The stomach must be mobilized with careful attention to the vascular supply on which the entire conduit depends. It is important to minimize trauma to the conduit, especially the most proximal portion, during mobilization. In a series of over 1,000 patients, Orringer and colleagues reported failure to adequately mobilize the gastric conduit (as opposed to improper anastomosis creation) as the most likely cause of gastric tube necrosis (5). The width of the conduit is also important. Pierie and associates demonstrated that creating a gastric tube which is too narrow can lead to fundal tip necrosis as a result of decreased mucosal blood flow (13). On the other hand a conduit which is too wide may become redundant within the chest or compressed as it passes through the thoracic inlet. Liebermann-Meffert and colleagues reported an ideal width of 4-5 cm in creating a gastric tube conduit (8).
Although several routes exist for placement of the neo-esophagus, the posterior mediastinal route allows for the best alignment and least tension on the conduit (14). Placement of the conduit in the substernal position may be used for patients with a hostile right thorax or mediastinum, but this route risks compression by the clavicular head. The subcutaneous position, used in only extreme conditions, is disadvantageous due to the longer length required and increased risk of conduit trauma (15). If the posterior mediastinal route is not available, then substernal should be the second choice with removal of the clavicular head and a portion of the manubrium to prevent compression.
Tension is also a key factor in preventing anastomotic complications. The length of the conduit available should be taken into account when choosing the level of the anastomosis. For example, in the case of a bulky gastroesophageal junction tumor requiring resection of a portion of the proximal stomach with the specimen, anastomosis in the neck may be under significant tension and a decreased chance of leak may be obtained by instead choosing an anastomosis within the chest. Some surgeons have attempted to reduce the tension on the anastomosis by tacking the conduit to the prevertebral fascia with sutures; however, this may increase the risk of anastomotic leak and gastric tube tip necrosis and should be avoided (16).
A variety of anastomotic techniques have been proposed to prevent leak and necrosis. Law et al. compared 1-layer hand sewn anastomoses to circular end-to-end stapled anastomoses and found no difference in leak rate (17). Heitmiller and colleagues describe using a two-layer hand sewn cervical anastomosis with an anastomotic leak rate of 0.8% (18). Orringer and colleagues favor a semi-mechanical anastomosis. An endoscopic linear stapler is used to create the back wall of a side-to-side esophagogastric anastomosis and the anterior wall is hand sewn in a single layer (19). Using this technique, they reported a significant decrease in their incidence of anastomotic leaks from 13% to 3%. Completely stapled anastomoses may be created using several techniques, including end-side on the anterior aspect of the stomach, end-side on the posterior aspect of the stomach, end-side with a circular stapler, and end-end with triangulated linear stapling. A recent retrospective study of the anastomotic strategies showed the highest leak rate with single layer hand sewn and the lowest stricture rate with the linear stapled technique (20). Despite the lack of consensus, an important technical aspect of all esophagogastric anastomoses is the incorporation of mucosa in order to provide adequate blood supply to the anastomosis.
The same risk factors which lead to the development of gastric conduit necrosis can generally be applied to the use of colon and jejunum with a few differences. Colon interposition requires the creation of at least three anastomoses instead of one, although the majority of ischemic complications are related to the esophagogastric anastomosis as is seen with the gastric conduit. Colon interpositions have an incidence of conduit necrosis reported as 2.4-18% which is much higher than that reported for gastric conduits (3,21,22). Similarly, jejunal conduits have necrosis rates reported up to 14.1% (23). Further differences particular to surgical technique in using colon and jejunum conduits are described below.
Postoperative management can also contribute to conduit ischemia. Postoperative hypotension has been reported to increase the risk of ischemia and ultimately necrosis (16). This may be exacerbated by some of the commonly used vasopressor agents which will cause splanchnic vasoconstriction. Conduit distention can also lead to decreased perfusion by increasing wall tension above the capillary perfusion pressure. Many surgeons routinely leave a nasogastric tube in the conduit at the completion of the procedure in an attempt to prevent this.
Diagnosis
The clinical presentation of conduit necrosis usually reflects mediastinitis and severe sepsis as sequelae of anastomotic leak and ischemic bowel. Signs and symptoms include fever, chest pain, tachycardia, tachypnea, oliguria, hypotension, acidosis and “coffee-grounds” nasogastric tube drainage. Conduit necrosis is usually an early postoperative event and rarely presents after the seventh postoperative day (24). As with any anastomotic leak, the key to diagnosis is having a high index of suspicion with any change in clinical status. What starts as simple tachycardia and fever can rapidly progress to hemodynamic instability and multi-organ system failure. Initial diagnostic maneuvers with any concern for anastomotic leak should include drainage which for a cervical esophageal anastomosis means opening the neck incision. If this does not resolve the fevers and systemic symptoms, endoscopy or operative exploration is indicated to confirm and manage conduit necrosis. Rapidly developing sepsis in combination with diffuse leakage on a contrast radiologic study mandates further investigation for conduit necrosis. If there is clinical suspicion with an intrathoracic anastomosis, a contrast esophagogram should be obtained to establish the presence of anastomotic leak. This may also demonstrate a mucosal cobblestone appearance characteristic of ischemia. Endoscopic evaluation of the conduit is the next step to evaluate the anastomosis and extent of graft necrosis. Evidence of mucosal ischemia or necrosis on endoscopy should prompt immediate surgical exploration. Endoscopy is a safe procedure with little risk of injury to the gastric conduit. Page and colleagues demonstrated that routine endoscopy in 100 patients within one week of esophagectomy posed no risk of injury to the gastric conduit or anastomosis (25). Computed tomography is a less useful test which can delineate a large anastomotic leak but often demonstrates only fluid and air within the mediastinum which may be normal postoperative findings and do not confirm a diagnosis of leak (26).
Management
With small anastomotic leaks, non-operative management is the strategy of choice. As long as there is adequate drainage and nutrition, the leak will likely heal. Conduit necrosis on the other hand mandates urgent surgical exploration. The conduit and anastomosis are examined, necrotic tissue including the conduit and surrounding mediastinum are debrided, and the area is widely drained. If the defect is not profound, consideration may be given to placing a t-tube to create a controlled fistula. If the defect is large with significant necrosis of the conduit, non-viable esophageal and gastric conduit tissue is resected and the remaining conduit is returned to the abdomen. The majority of patients will have signs of sepsis including hemodynamic instability and the conservative and most commonly used approach includes proximal diversion. Reconstruction with a new conduit in the same setting is not recommended (27). Proximal diversion by creation of a temporary cervical esophagostomy and feeding jejunostomy allows sepsis to resolve prior to future esophageal reconstruction. The longest possible length of remaining esophagus should be preserved in creating a temporary esophagostomy to allow for an easier future reconstruction of gastrointestinal continuity.
The most common options for esophageal reconstruction following necrosis of a gastric conduit include colon interposition or jejunal transfer as a free or “supercharged” conduit. Advantages to using a colon interposition include its fairly consistent arterial supply, and the ability to replace long segments of necrotic esophageal conduit. Disadvantages include the possibility of intrinsic vascular disease and the need for three anastomoses. Advantages to using jejunum as conduit include its similar width size match to the esophagus, arterial supply largely spared of intrinsic vascular disease, and active peristalsis to assist in food bolus transit. The use of jejunum conduit is less popular than colon, however, because its vascular anatomy limits its use to short-segment esophageal replacement. A jejunum conduit which has been “supercharged” by microvascular augmentation has been shown to reduce incidence of ischemic complications and achieve long-segment esophageal reconstruction, however this more technically demanding operation has not gained favor among most surgeons (28).
Outcomes
Although conduit necrosis is rare, it can be a disastrous complication. Hospital mortality has been reported approaching 90% especially if the necrosis is not diagnosed promptly (4). Iannettoni and colleagues reported a series of six patients with gastric tip necrosis. Two of the six (33%) died during that hospital stay (16). Schuchert et al. reported a similar rate with one of three patients with gastric tip necrosis dying in the perioperative period (29). Although they were able to preserve the conduit, both of their survivors ended up with strictures requiring multiple dilations.
Conduit airway fistula
A benign fistula between the trachea and the neo-esophagus following esophagectomy is a rare but potentially fatal complication. Conduit airway fistula has a reported incidence of 0.04-0.3% and, like conduit necrosis, tends to present relatively early in the postoperative course (30). Fistula formation is possible due to the close anatomic relationship of the conduit and the airway. If the esophagectomy is done using the Ivor Lewis technique, the anastomosis lies just cephalad to the azygous vein directly posterior to the membranous airway. A fistula at this level will potentially involve the distal trachea, carina, right main bronchus or left main bronchus. With a transhiatal or McKeown approach, the anastomosis is in the cervical esophagus which lies behind and slightly to the left of the membranous airway. Fistulas can also occur at levels other than the anastomosis. Fistulas can originate from the gastric staple line which runs the length of the conduit, from old feeding tube sites or from a penetrating gastric ulcer (31-34).
Etiology
Conduit airway fistula generally occurs in the setting of anastomotic leak. The local inflammation of the leaking enteric contents and saliva cause necrosis of the surrounding tissue which can erode into the airway. There may or may not be an underlying airway injury as well which creates a weak point susceptible to fistula creation. The trachea is most commonly injured during mobilization of the esophagus within the chest, either by direct trauma or injudicious use of an energy source (such as electrocautery). This is particularly likely when the tumor is at or above the level of the carina (35). Unsuspected airway injuries can also occur during intubation. Extensive dissection around the trachea at the level of the carina can interrupt the local blood supply and lead to ischemia. Fistulization has been reported to be particularly related to devascularization of the membranous trachea overlying the esophagus as a result of radical upper mediastinal lymph node dissection (36). Maruyama and colleagues demonstrated a relationship between conduit-airway fistula and three field lymph node dissection or a total lymph node count of greater than 60 nodes (37). Airway injury has also been reported due to chronic irritation from the gastric staple line running the length of the conduit. Neoadjuvant therapy can lead to preexisting tissue injury/ischemia. Heitmiller and colleagues identified a correlation between neoadjuvant chemotherapy and increased risk of neo-esophageal fistula development (18). Bartels and colleagues found an even stronger association with neoadjuvant radiation (35).
Even without an underlying airway injury, an inadequately drained anastomotic leak will cause inflammation with local release of gastric enzymes and may fistulize into the airway. Another more chronic issue which may lead to fistulas is pressure created by the cuff of an endotracheal or tracheostomy tube in a patient requiring mechanical ventilation for a prolonged period postoperatively (38,39). Patients who successfully heal an anastomotic leak may require long term treatment for a resulting stricture. The more liberal use of stents to treat anastomotic stricture after leak has led to case reports of stent erosion into the airway with resulting fistula (40). Fistula has also been reported after endoscopic dilation of anastomotic stricture (30).
Diagnosis
The clinical presentation spectrum varies from mild disease to life-threatening sepsis. The most common early symptom of fistula is cough with oral intake and shortness of breath due to aspiration of gastric contents. This can progress to recurrent aspiration pneumonias and sepsis. Some patients will present early in the development with relatively few symptoms while others present with acute decompensation related to chemical with or without superimposed bacterial pneumonitis and pneumonia. An esophagram may demonstrate oral contrast entering the airway although if the fistula is small the study may be nondiagnostic. If the clinical suspicion is high or the esophagram shows a fistula endoscopic confirmation is necessary. This should include endoscopic inspection of the upper esophagus, anastomosis, conduit and airway. The anastomosis and gastric staple line are common sites of leak and should be inspected carefully but it is often difficult to identify a small fistula from the esophageal side. The folded mucosa of the conduit may hide a small opening. The fistula is often better identified from the airway side where the size and location should be noted. Unless the patient is in the early postoperative period, it is important to biopsy the fistula to determine if it is due to recurrent cancer or is truly a benign lesion. If it is still in the early postoperative period, a CT scan may be useful to identify surrounding fluid collections which will need to be drained. The fistula itself can occasionally be identified on a CT scan (Figure 1) but this should not be relied upon for diagnosis as it is neither sensitive nor specific.
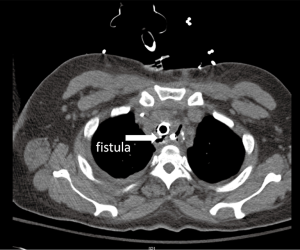
Management
Optimal management of conduit airway fistulas should be dictated by the location and size of the fistula in conjunction with the severity of the patient’s symptoms. In a patient presenting with minimal symptoms, endoscopic approaches to repair are a reasonable first choice. Many endoscopic techniques have been tried to directly close the fistula including application of fibrin glue with or without a vicryl mesh plug and metallic clip placement (41). These approaches are more likely to work with a long narrow fistula tract and good results have been reported (42,43). Unfortunately, even in the ideal fistula, this approach may be unsuccessful at obliterating the leak or lead to early recurrence (44). Another endoscopic approach is to cover the fistula with a self-expanding stent which may allow the surrounding tissue to remodel and scar over the opening. Boyd and Rubio reviewed the published experience with this technique and concluded that covered metallic stents may be more successful at occluding the fistula initially and preventing further airway soilage but there was no difference in long term success (45). Initial closure was successful in 75% of the cases they reviewed. Most failures were due to leakage around the stent related to an imperfect seal. It is often difficult to stent from the conduit side because of the large diameter of the conduit, which leads to both stent migration as well as reflux around the distal end of the stent. This is a more significant issue if the fistula is with the body of the conduit rather than at the anastomosis. The airway side of the fistula is often a more feasible diameter to allow good contact with the wall circumferentially. Stenting has a high recurrence rate (39%) and the patients must be monitored carefully for recurrence and extension. There is a concern that the radial force of an oversized stent will create local tissue ischemia and actually enlarge the fistula rather than allowing it to heal. More recently, several studies have demonstrated the feasibility of using dual self-expandable stents placed by endoscopy and tracheoscopy in the alimentary and respiratory tracts for benign and malignant fistulas following esophagectomy (46). Although this approach may improve initial closure rates, the tissue between the stents then becomes even more susceptible to pressure ischemia and necrosis. While this stent-based strategy has had variable success in fistula closure, it is a useful tool for temporizing the patient who presents acutely ill. Occlusion of the fistula allows treatment of associated pneumonia and nutritional optimization as needed and facilitates a safer, elective reconstruction with single-stage repair instead of an urgent surgical intervention. A final experimental endoscopic approach which has been reported for use in fistulas recurrent after both endoscopic and surgical closure is placement of a cardiac septal occluder (47). This device is delivered over a wire using the gastroscope and consists of two self-expanding nitinol discs joined at their centers. The link between the two sides sits across the fistula with a disc providing occlusion from either side.
Surgical intervention requires an individualized approach based on the location of the fistula and quality of the surrounding tissue. Successful repair can be achieved with adherence to the following principles: drainage and debridement of non-viable tissue, primary repair of the tracheal and conduit defects, and interposition of well vascularized tissue between the trachea and esophagus to prevent recurrent fistulization. Many sources of vascularized tissue have been successfully used including omentum, pericardium, pleura, pericardial fat pad, and intercostal muscle. The tracheal defect can be closed primarily if small enough not to compromise the lumen. If it is too large the membranous portion of the airway can be reconstructed with either autologous tissue or biologic mesh and then reinforced with vascularized tissue. Bakhos and colleagues were successful with primary repair of a fistula buttressed with an intercostal muscle flap (31). Kron and colleagues describe using pericardial patch to replace membranous trachea followed by interposition of a latissimus dorsi flap to isolate the gastric conduit from the trachea (32). If inadequate local tissue is available, the use of biologic mesh with a reinforced interposition flap may be helpful as described by Reames and colleagues (48). Since the conduit has more flexibility and redundancy than the airway it can most often be closed primarily. In rare circumstances the gastric conduit may be deemed nonviable, in which case it should be excised and managed as for gastric conduit necrosis. In their series of six patients with benign trachea-neo-esophageal fistulas following esophagectomy, Buskens and colleagues report two patients who were treated conservatively, one patient who had a fistula partly excised via a right sided cervical incision, and three patients who underwent partial exclusion or excision of the gastric conduit followed by colonic interposition reconstruction with good results (30). A staged reconstruction with proximal esophageal diversion followed by delayed re-establishment with the colon should be used if esophageal continuity is disrupted.
Summary
Prevention of ischemic complications can be best achieved by early identification of potential patient risk factors, careful conduit mobilization during surgery, and diligent postoperative care. Diagnosis relies on a high index of suspicion in patients with unusual findings in their postoperative course such as unexplained sepsis and recurrent pneumonias or cough with oral intake. Conduit necrosis mandates surgical intervention with aggressive debridement of nonviable tissue and will often require esophageal diversion with staged reconstruction after controlling mediastinal sepsis. Conduit airway fistulas are more variable in their clinical presentation making diagnosis challenging. The size and location of the defect in conjunction with the severity of symptoms should dictate the appropriate management. Despite optimal identification and management of these complications, mortality rates are high and survivors can expect a prolonged course with multiple reinterventions.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Lerut T, Coosemans W, Decker G, et al. Anastomotic complications after esophagectomy. Dig Surg 2002;19:92-8. [PubMed]
- Ferri LE, Law S, Wong KH, et al. The influence of technical complications on postoperative outcome and survival after esophagectomy. Ann Surg Oncol 2006;13:557-64. [PubMed]
- Wormuth JK, Heitmiller RF. Esophageal conduit necrosis. Thorac Surg Clin 2006;16:11-22. [PubMed]
- Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg 1995;169:634-40. [PubMed]
- Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg 1999;230:392-400; discussion 400-3. [PubMed]
- Ramage L, Deguara J, Davies A, et al. Gastric tube necrosis following minimally invasive oesophagectomy is a learning curve issue. Ann R Coll Surg Engl 2013;95:329-34. [PubMed]
- Akiyama H. Esophageal anastomosis. Arch Surg 1973;107:512-4. [PubMed]
- Liebermann-Meffert DM, Meier R, Siewert JR. Vascular anatomy of the gastric tube used for esophageal reconstruction. Ann Thorac Surg 1992;54:1110-5. [PubMed]
- Cassivi SD. Leaks, strictures, and necrosis: a review of anastomotic complications following esophagectomy. Semin Thorac Cardiovasc Surg 2004;16:124-32. [PubMed]
- Dewar L, Gelfand G, Finley RJ, et al. Factors affecting cervical anastomotic leak and stricture formation following esophagogastrectomy and gastric tube interposition. Am J Surg 1992;163:484-9. [PubMed]
- Peracchia A, Bardini R, Ruol A, et al. Esophagovisceral anastomotic leak. A prospective statistical study of predisposing factors. J Thorac Cardiovasc Surg 1988;95:685-91. [PubMed]
- Sabel MS, Smith JL, Nava HR, et al. Esophageal resection for carcinoma in patients older than 70 years. Ann Surg Oncol 2002;9:210-4. [PubMed]
- Pierie JP, de Graaf PW, van Vroonhoven TJ, et al. The vascularization of a gastric tube as a substitute for the esophagus is affected by its diameter. Dis Esophagus 1998;11:231-5. [PubMed]
- Gupta NM, Gupta R. Transhiatal esophageal resection for corrosive injury. Ann Surg 2004;239:359-63. [PubMed]
- Perez M, Haumont T, Arnoux JM, et al. Anatomically based comparison of the different transthoracic routes for colon ascension after total esogastrectomy. Surg Radiol Anat 2010;32:63-8. [PubMed]
- Iannettoni MD, Whyte RI, Orringer MB. Catastrophic complications of the cervical esophagogastric anastomosis. J Thorac Cardiovasc Surg 1995;110:1493-500; discussion 1500-1. [PubMed]
- Law S, Fok M, Chu KM, et al. Comparison of hand-sewn and stapled esophagogastric anastomosis after esophageal resection for cancer: a prospective randomized controlled trial. Ann Surg 1997;226:169-73. [PubMed]
- Heitmiller RF, Fischer A, Liddicoat JR. Cervical esophagogastric anastomosis: results following esophagectomy for carcinoma. Dis Esophagus 1999;12:264-9. [PubMed]
- Orringer MB, Marshall B, Iannettoni MD. Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 2000;119:277-88. [PubMed]
- Price TN, Nichols FC, Harmsen WS, et al. A comprehensive review of anastomotic technique in 432 esophagectomies. Ann Thorac Surg 2013;95:1154-60; discussion 1160-1. [PubMed]
- Davis PA, Law S, Wong J. Colonic interposition after esophagectomy for cancer. Arch Surg 2003;138:303-8. [PubMed]
- DeMeester TR, Johansson KE, Franze I, et al. Indications, surgical technique, and long-term functional results of colon interposition or bypass. Ann Surg 1988;208:460-74. [PubMed]
- Moorehead RJ, Wong J. Gangrene in esophageal substitutes after resection and bypass procedures for carcinoma of the esophagus. Hepatogastroenterology 1990;37:364-7. [PubMed]
- Dowson HM, Strauss D, Ng R, et al. The acute management and surgical reconstruction following failed esophagectomy in malignant disease of the esophagus. Dis Esophagus 2007;20:135-40. [PubMed]
- Page RD, Asmat A, McShane J, et al. Routine endoscopy to detect anastomotic leakage after esophagectomy. Ann Thorac Surg 2013;95:292-8. [PubMed]
- Oezcelik A, Banki F, Ayazi S, et al. Detection of gastric conduit ischemia or anastomotic breakdown after cervical esophagogastrostomy: the use of computed tomography scan versus early endoscopy. Surg Endosc 2010;24:1948-51. [PubMed]
- Paul S, Bueno R. Section VI: complications following esophagectomy: early detection, treatment, and prevention. Semin Thorac Cardiovasc Surg 2003;15:210-5. [PubMed]
- Heitmiller RF, Gruber PJ, Swier P, et al. Long-segment substernal jejunal esophageal replacement with internal mammary vascular augmentation. Dis Esophagus 2000;13:240-2. [PubMed]
- Schuchert MJ, Abbas G, Nason KS, et al. Impact of anastomotic leak on outcomes after transhiatal esophagectomy. Surgery 2010;148:831-8; discussion 838-40. [PubMed]
- Buskens CJ, Hulscher JB, Fockens P, et al. Benign tracheo-neo-esophageal fistulas after subtotal esophagectomy. Ann Thorac Surg 2001;72:221-4. [PubMed]
- Bakhos C, Alazemi S, Michaud G, et al. Staged repair of benign tracheo-neo-esophageal fistula 12 years after esophagectomy for esophageal cancer. Ann Thorac Surg 2010;90:e83-5. [PubMed]
- Kron IL, Johnson AM, Morgan RF. Gastrotracheal fistula: a late complication after transhiatal esophagectomy. Ann Thorac Surg 1989;47:767-8. [PubMed]
- Saito H, Minamiya Y, Hashimoto M, et al. Repair of reconstructed gastric tube bronchial fistulas after operation for esophageal cancer by transposing a pedicled pectoralis major muscle flap: report of three successful cases. Surgery 1998;123:365-8. [PubMed]
- Hayashi K, Ando N, Ozawa S, et al. Gastric tube-to-tracheal fistula closed with a latissimus dorsi myocutaneous flap. Ann Thorac Surg 1999;68:561-2. [PubMed]
- Bartels HE, Stein HJ, Siewert JR. Tracheobronchial lesions following oesophagectomy: prevalence, predisposing factors and outcome. Br J Surg 1998;85:403-6. [PubMed]
- Fujita H, Kawahara H, Hidaka M, et al. An experimental study on viability of the devascularized trachea. Jpn J Surg 1988;18:77-83. [PubMed]
- Maruyama K, Motoyama S, Sato Y, et al. Tracheobronchial lesions following esophagectomy: erosions, ulcers, and fistulae, and the predictive value of lymph node-related factors. World J Surg 2009;33:778-84. [PubMed]
- Kalmár K, Molnár TF, Morgan A, et al. Non-malignant tracheo-gastric fistula following esophagectomy for cancer. Eur J Cardiothorac Surg 2000;18:363-5. [PubMed]
- Nordin U. The trachea and cuff-induced tracheal injury. An experimental study on causative factors and prevention. Acta Otolaryngol Suppl 1977;345:1-71. [PubMed]
- Shichinohe T, Okushiba S, Morikawa T, et al. Salvage of a massive esophago-tracheal fistula resulting from a stenting treatment. Dis Esophagus 2006;19:299-304. [PubMed]
- Truong S, Böhm G, Klinge U, et al. Results after endoscopic treatment of postoperative upper gastrointestinal fistulas and leaks using combined Vicryl plug and fibrin glue. Surg Endosc 2004;18:1105-8. [PubMed]
- Ng WT, Luk HT, Lau CW. Endoscopic treatment of recurrent tracheo-oesophageal fistulae: the optimal technique. Pediatr Surg Int 1999;15:449-50. [PubMed]
- Marone G, Santoro LM, Torre V. Successful endoscopic treatment of GI-tract fistulas with a fast-hardening amino acid solution. Endoscopy 1989;21:47-9. [PubMed]
- Nardella JE, Van Raemdonck D, Piessevaux H, et al. Gastro-tracheal fistula--unusual and life threatening complication after esophagectomy for cancer: a case report. J Cardiothorac Surg 2009;4:69. [PubMed]
- Boyd M, Rubio E. The utility of stenting in the treatment of airway gastric fistula after esophagectomy for esophageal cancer. J Bronchology Interv Pulmonol 2012;19:232-6. [PubMed]
- Elbe P, Lindblad M, Tsai J, et al. Non-malignant respiratory tract fistula from the oesophagus. A lethal condition for which novel therapeutic options are emerging. Interact Cardiovasc Thorac Surg 2013;16:257-62. [PubMed]
- Repici A, Presbitero P, Carlino A, et al. First human case of esophagus-tracheal fistula closure by using a cardiac septal occluder (with video). Gastrointest Endosc 2010;71:867-9. [PubMed]
- Reames BN, Lin J. Repair of a complex bronchogastric fistula after esophagectomy with biologic mesh. Ann Thorac Surg 2013;95:1096-7. [PubMed]


