
Cardio-toxicity in childhood cancer survivors “Cure is not enough”
Background
According to the latest publication of data by the German Children’s cancer registry (DKKR), the 5-year survival rate for children aged under 15 years is estimated with 85%. Since 1980, 47,650 patients have been documented (1). Attention is drawn towards cardiac or cardiovascular sequelae in childhood cancer survivors. Accordingly, this topic was covered in adult cancer survivor studies leading to the ESC position paper on cardiac toxicity, quoted in the subtitle mentioned above (2). There are publications on chemotherapy-induced cardiotoxicity (mainly anthracycline-associated) and additional risk factors. Also radiation, chronic disease, combination of chemotherapies and the introduction of new drugs need to be considered (3). Furthermore, asymptomatic (subclinical) or symptomatic cardiac events have to be distinguished (4). Asymptomatic ventricular dysfunction has been reported to be as high as 57% (5,6). The appearance of cardiomyopathy is reported from 2.8% to 29%, mostly in accordance to-the dose of anthracyclines (7,8).
Several population based registries or studies were reporting on late term problems of childhood cancer survivors and cardiovascular sequelae.
The British Childhood Cancer Survivor Study (BCCSS) (9) represented a cohort of 34,489 5-year survivors of childhood cancer diagnosed between 1940 and 2006. They counted 181 cardiac deaths, a number 3.4 times higher than expected. In the group of participants of age 60 years and older, cardiac disease was present in 22% and vascular sequelae in 15% of the cases. Surprisingly, the mortality was highest among the patients diagnosed between 1980 and 1989.
The Euro2K cohort followed 4,567 2-year survivors of childhood cancer treated between 1942 and 1985 (10). Medical archives and cancer centers in France and UK were explored. A total of 3,162 5-year survivors were analyzed. Medium follow-up was 26 years. Heart radiation dose was reconstructed. And 234 participants showed manifestations of cardiac disease. Heart failure was present in 38% of these cases, valvular disease in 23%, arrhythmia and conduction disorders in 23%. The highest and earliest risk factors for cardiac disease were identified as therapy with anthracycline and radiation >15 Gy. One conclusion was that “survivors should be aware of the risk of late cardiac disease and should be counseled to avoid other cardiac risk factors”.
In the Scandinavian cohort (ALiCCS), 32,308 1-year survivors diagnosed before the age of twenty were included (11). Registration started in the 1940s and 1950s and ended in 2008. At closure of follow-up, 12% of the participants were older than 50 years of age, 4.5% over 60. The highest relative risks were seen for heart failure, valvular dysfunction and cerebrovascular disease.
Late mortality was retrospectively examined in the CCSS in the US (12). Data of 20,227 5-year survivors treated between 1970 and 1986 were analyzed. They reported 2,030 deaths before 1996. This exceeded the expectations 11 fold. In 83 cases (4.5%), death was attributed to cardiac toxicity.
The German CVSS-study examined 1,002 individuals with cancer before the age of 15, diagnosed between 1980 and 1990 (13). They all were well documented in the German registry. As a control group they included a single centre-cohort of 15,000 people in the region of Mainz. The examination protocol was a highly standardized evaluation form of clinical examinations and designed to detect risk factors for cardiovascular disease. At closure, 951 participants were analyzed, mean 28.4 years after diagnosis. Highest prevalence of risk factors was found for arterial hypertension (23%) and dyslipidemia (28.3%). Cardiovascular disease was prevalent in 4.5% of the participants and was elevated twice in comparison to the control group.
As a consequence, we have to identify individuals at risk, develop preventive strategies, and promote early detection of cardiovascular disease, early and/or preventive treatment by follow up throughout a lifetime. The recent knowledge will be discussed, followed by proposals for the future.
Important issues
Identify individuals at risk
There is a general elevated risk for cardiovascular disease in patients undergoing cancer treatment. Even though radiation and anthracycline toxicity, alone or combined, seem to be of primary importance (10), also young age below 4 years and female sex are associated with a higher risk of toxicity. Combinations with other cardiotoxic agents as e.g., cyclophosphamide, ifosfamide, fluorouracil, bleomycin, vincristine, mitoxantrone, trastuzumab are prone to increase the risk. Additional underlying cardiovascular risk factors like hypertension, obesity, diabetes, dyslipidemia, imbalanced life-style, low physical exercise, alcohol, smoking and stress lead to further deterioration. Furthermore an individual, genetically determined variability needs to be taken into account (Table 1).

Full table
Prevention of anthracycline- induced cardiotoxicity
Anthracycline-induced cardiotoxicity (symptomatic vs asymptomatic) is the leading topic throughout the publications. Risk factors accounting for toxicity are a high cumulative dosage, high single dose or bolus injections, other preexisting cardiac diseases, radiation in the region of the heart, young age, poor general condition. Acute or subacute toxicity (with signs of peri-myocarditis, rhythm disturbances-usually reversible and transient) and chronic damage, early-onset or chronic progressive late-onset cardiotoxicity (more than 1 year, loss of cardiomyocytes, irreversible) are distinguished. Cardiac failure and cardiomyopathy are known as the end stage of the clinical course. Up to 45% of patients receiving anthracyclines showed echocardiographic changes, heart failure was present in 10% of patients receiving more than 300 mg/m2 of cumulative anthracyclines. However, variability in terms of reaction to a certain cumulative dosage seems to be apparent as some patients tolerated more than 1,000 mg/m2 anthracyclines without signs of toxicity, whereas some developed cardiomyopathy at a cumulative dosage below <200 mg/m2. Estimates for a completely safe dosage of anthracyclines cannot be given and there are publications suggesting individual genetic dispositions (5,14). Studies regarding the different aspects demonstrated significant long term risks at a cumulative dose of >500 mg/m2.
Anthracycline-induced cardiotoxicity still is not completely understood and multiple mechanisms are involved. One theory is that reactive oxygen species and free radical formation directly trigger cardiac damage. The potential damage anthracyclines might cause in several structural proteins in the myocardium, e.g., Titin, is another aspect. Furthermore, dystrophin may be a target. Its damage may increase the risk of dilated cardiomyopathy. The expression of mitochondrial RNA is reduced and mitochondrial DNA will be damaged. Signaling proteins in the cardiovascular system also may play a role. The ErbB2 signaling pathway and the growth factor neuroregulin are of much interest.
Preventive strategies were mainly infusion time, liposomal formula and—since the 1980’s—the use of dexrazoxane.
Whether anthracyclines should be administered as a quick short bolus or slow rate infusion is also subject of further discussion. Myocardial damage does not only seem to depend on the cumulative dose but also on peak serum levels. As a consequence, long-term continuous infusion was recommended. In some protocols long-term infusion >48 h was proposed (15). In a Cochrane analysis (16), 11 studies were identified, 7 evaluated different infusion durations and 4 different peak doses. The meta-analysis showed a statistically lower rate of clinical heart failure with an infusion duration of 6 hours or longer. However, the number of participants enrolled in these studies was small. The most recent publication (17) aimed to give recommendations on an infusion duration. The panel was not able to recommend a specific duration of infusion, though they suggested a favourable infusion duration >1 hour. To answer this question, randomized trials will be required.
Liposomal-encapsulated anthracyclines have been developed to reduce toxicity. These intravenously administered drugs cannot escape the vascular space in sites with tight capillary junctions, which for example are found in the heart muscle. Upon the assumption of the liposomal formula being less cardiotoxic, a systematic review was performed (18). The literature did not show any randomized controlled trial or controlled clinical trial. Only 15 observational studies described the use in children with cancer, thus there were no recommendations given on the use of liposomal anthracyclines or even proof of their advantages.
Q10 coenzyme, vitamin E or carnitine had been introduced as agents with cardioprotective properties, whereas Dexrazoxane was used because of its effect as iron chelator and inhibitor of free radical formation in the heart. Studies supported the hypothesis that dexrazoxane was protective. In patients with either T-cell ALL or lymphoblastic non-Hodgkin-lymphoma, dexrazoxane was given in 273 out of 537. The mean left ventricular fractional shortening, wall thickness and thickness-to-dimension ratio showed worse results in the doxorubicin-alone group (19). However, other reports were published indicating that this substance may reduce the efficacy of therapy (20). Until 2017 therefore the recommendation of the EMA was to use dexrazoxane only in adult patients with advanced metastatic breast cancer-who had already received high cumulative doses of anthracyclines. A recommendation for the use in children was not given due to concerns of an increased risk of secondary malignancies (21).
A reevaluation (22) of published data leads to a removal of the general contraindication in children. Now children aged 0–18 years could receive dexrazoxane if the expected cumulative dose will be more than 300 mg/m2 doxorubicin or the equivalent of other anthracyclines.
Early detection of cardiovascular disease, dysfunction vs. failure
The cardiac damage of anthracyclines is a continuum from asymptomatic structural or functional abnormalities detected on imaging studies, to clinically symptomatic heart failure. In case of heart failure diagnosis and treatment should follow the established guidelines (23,24). Early detection of cardiac anomalies should be the goal allowing for preventive treatment in the asymptomatic setting (25). Clinical care guidelines have been established which recommend routine echocardiographic screening (26).
However, there are several questions left unanswered:
- How long should patients be screened?
- Do low-risk survivors benefit from the screening?
- Is there a benefit in early interventions?
Cardiac biomarkers like troponin were not helpful in detecting chronic injury. Also natriuretic peptides did not meet screening criteria, but could be helpful to exclude heart failure (27).
The most important diagnostic tools certainly lie within the different imaging methods, such as echocardiography, but also MRI, radionuclide angiography, SPECT or PET. Echocardiography is widely used in daily routine. Left ventricular fractional shortening (SF) and ejection fraction (EF) are established parameters to quantify systolic left ventricular function, but changes of 10% are necessary to reach a level of significance. EF in transthoracic echocardiography is overestimated compared to EF measurements in cardiac MRI (28).
Looking at diastolic function is of high importance, too. In cases with diastolic heart failure systolic function is preserved. Within the St. Jude lifetime cohort study 3D-EF, global longitudinal and circumferential myocardial strain and diastolic function were examined (29). The 5.8% of 1,820 adults enrolled after cancer therapy had abnormal 3D-EF, but 28% of the participants showed changes in global strain and/or diastolic function parameters.
Early and/or preventive treatment: secondary prophylaxis
Detecting cardiac dysfunction should have a consequence. Several publications emphasized the application of drugs used in heart failure therapy (30,31). One study in particular focused on the effect of enalapril in cardiomyopathy, independent from the underlying disease. They could show a higher reduction in mortality due to cardiovascular events in the enalapril group, though their results were not statistically significant (32).
There are reports on the prophylactic use of ACE-inhibitors in childhood cancer survivors. A treatment with enalapril had short term beneficial effects but also caused side effects. It did not prevent progressive LV wall thinning (33). However, this study was designed in retrospective and involved 18 patients only.
In a randomized trial with 69 participants treated with enalapril versus 66 participants in a placebo group (34) exercise testing and echocardiography were performed. There was no influence on exercise performance but reduction of left ventricular end-systolic wall stress, which however proofed to be transient. There still is a need for evidence (35).
Follow up throughout a lifetime-monitoring of cardiac function
In 1992 (26), guidelines for monitoring cardiotoxicity during anthracycline therapy were published. The basis was published literature, established methods and the experience of the author. Echocardiography or radionuclide angiocardiography was used imaging cardiac function. EF and left ventricular SF were measured. SF <29%, EF <55% or drop in the absolute value of ≥10% were defined as signs of toxicity. They proposed baseline ECG and Echo, during therapy and after. This surely is not enough, but often the standard in follow up. Recommendations regarding cardiac monitoring included in collaborative European multicenter trial protocols were evaluated in terms of efficacy (36). A wide variation of different cardiac monitoring routines was found in these protocols. In consequence, there is a strong need of evidence from clinical research and it is important to uniform cardiac monitoring schedules.
In 2008 (37), a multidisciplinary task force proposed an assessment including 6 different categories:
- Identifying the individual risk regarding cumulative dose and type of anthracycline used, field and dose of radiation and age at time of therapy;
- Additional cardiovascular risk factors e.g., dyslipidemia, hypertension, obesity, smoking, family history;
- Detailed review of symptoms;
- Monitoring cardiac function, always with the same method;
- Counseling regarding lifestyle;
- Monitoring of cardiac function during pregnancy and/or general anesthesia.
Baseline echo-examination and ECG for QTc were mandatory, and then depending on risk factors. Asymptomatic low-risk patients (>5 years, <250 mg/m2, no radiotherapy) should be seen once a year. There were no recommendations given for detailed examinations.
Effort was put into harmonizing this on an international level (25). Definition of risk groups in low (Anthracycline dose <100 mg/m2), moderate (anthracycline dose 100 to <250 mg/m2 or chest radiation ≥15 to <35 Gy) and high (anthracycline >250 mg/m2 or chest radiation ≥35 Gy and combination of anthracycline ≥100 and radiation ≥15 Gy) was agreed on. The recommendations were graded according to the quality of evidence. Cardiomyopathy surveillance should start no later than 2 years after completion of cardiotoxic therapy and repeated every 5 years.
Conclusions
Cardiotoxicity is a serious complication of cancer therapy. Mortality in the long-term is about 10 fold higher compared to age-matched control groups. There is a constant increase of heart failure with age. Main reasons are anthracyclines and radiation. There needs to be a balance in therapeutic efficacy and toxicity. According to the literature these statements can be done.
Infusion time with anthracyclines should be more than 1 hour.
Dexrazoxane can be administered in children expecting >300 mg/m2 doxorubicin or an anthracycline equivalent.
Patients at risk need to be characterized more thoroughly.
Echocardiographic examination routines need to be improved and implemented in the ongoing studies. Standard 2D-SF and EF should be complemented by additional techniques such as strain, 3D-Echo and diastolic parameters. A practical proposal in Germany is in preparation.
Secondary prophylaxis with enalapril alone does not seem to be beneficial in terms of outcome.
Further studies prospective regarding the use of β-blocking agents and combination of drugs should be designed.
Lifelong awareness of cardiovascular damage/anomalies/changes should be considered mandatory, at least for high risk patients (Table 2).
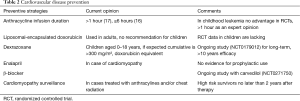
Full table
The detection of cardiac problems will probably increase with a stricter follow-up. In the future, we might not only have to expect cardiomyopathies, but also valvular and pericardial damage, rhythm disturbances, arterial hypertension, vasculopathy, stroke and metabolic disorders.
The burden of cardiovascular morbidity will accompany childhood cancer survivors throughout their whole lifetime. It already is known as the leading cause of non-cancer-related morbidity and mortality.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Scholz-Kreisel P, Kaatsch P, Spix C, et al. Second malignancies following childhood cancer treatment in Germany from 1980 to 2014 – a registry-based analysis. Dtsch Arztebl Int 2018;115:385-92. [PubMed]
- Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768-801. [Crossref] [PubMed]
- Armenian SH, Armstrong GT, Aune G, et al. Cardiovascular Disease in Survivors of Childhood Cancer: Insights into Epidemiology, Pathophysiology, and Prevention. J Clin Oncol 2018;36:2135-44. [Crossref] [PubMed]
- van der Pal HJ, van Dalen EC, van Delden E, et al. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol 2012;30:1429-37. [Crossref] [PubMed]
- Lipshultz SE, Colan SD, Gelber RD, et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med 1991;324:808-15. [Crossref] [PubMed]
- Kremer LC, van der Pal HJ, Offringa M, et al. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol 2002;13:819-29. [Crossref] [PubMed]
- Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions:a scientific statement from the American Heart Association. Circulation 2013;128:1927-95. [Crossref] [PubMed]
- Armenian SH, Hudson MM, Mulder RL, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet oncol 2015;16:e123-36. [Crossref] [PubMed]
- Fidler MM, Reulen RC, Henson K, et al. Population-Based Long-Term Cardiac-Specific Mortality among 34 489 Five-Year Survivors of Childhood Cancer in Great Britain. Circulation 2017;135:951-63. [Crossref] [PubMed]
- Haddy N, Diallo S, El-Fayech C, et al. Cardiac Diseases Following Childhood Cancer Treatment: Cohort Study. Circulation 2016;133:31-8. [Crossref] [PubMed]
- Gudmundsdottir T, Winther JF, de Fine Licht S, et al. Cardiovascular disease in Adult Life after Childhood Cancer in Scandinavia: A population-based cohort study of 32,308 one-year survivors. Int J Cancer 2015;137:1176-86. [Crossref] [PubMed]
- Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol 2001;19:3163-72. [Crossref] [PubMed]
- Faber J, Wingerter A, Neu MA, et al. Burden of cardiovascular risk factors and cardiovascular disease in childhood cancer survivors: data from the German CVSS-study. Eur Heart J 2018;39:1555-62. [Crossref] [PubMed]
- Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart 2008;94:525-33. [Crossref] [PubMed]
- Sadurska E. Current Views on Anthracycline Cardiotoxicity in Childhood Cancer Survivors. Pediatr Cardiol 2015;36:1112-9. [Crossref] [PubMed]
- van Dalen EC, van der Pal HJ, Kremer LC. Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Cochrane Database Syst Rev 2016;3:CD005008. [PubMed]
- Loeffen EAH, van Dalen EC, Mulder RL, et al. The duration of anthracycline infusion should be at least one hour in children with cancer: A clinical practice guideline. Pediatr Blood Cancer 2018;65. [Crossref] [PubMed]
- Sieswerda E, van Dalen EC, Postma A, et al. Medical interventions for treating anthracycline-induced symptomatic and asymptomatic cardiotoxicity during and after treatment for childhood cancer. Cochrane Database Syst Rev 2011;7:CD008011. [PubMed]
- Asselin BL, Devidas M, Chen L, et al. Cardioprotection and Safety of Dexrazoxane in Patients Treated for Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or Advanced-Stage Lymphoblastic Non-Hodgkin Lymphoma:A Report of the Children's Oncology Group Randomized Trial Pediatric Oncology Group 9404. J Clin Oncol 2016;34:854-62. [Crossref] [PubMed]
- Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol 2007;25:493-500. [Crossref] [PubMed]
- Kremer LC, van Dalen EC. Dexrazoxane in Children With Cancer: From Evidence to Practice. J Clin Oncol 2015;33:2594-6. [Crossref] [PubMed]
- Reichardt P, Tabone MD, Mora J, et al. Risk-benefit of dexrazoxane for preventing anthracycline-related cardiotoxicity: re-evaluating the European labeling. Future Oncol 2018;14:2663-76. [Crossref] [PubMed]
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14:803-69. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016;134:e282-93. [PubMed]
- Armenian SH, Wong FL. Screening for Anthracycline-Related Cardiac Dysfunction in Childhood Cancer Survivors: Can Less be More? Pediatr Blood Cancer 2015;62:2067-8. [Crossref] [PubMed]
- Steinherz LJ, Graham T, Hurwitz R, et al. Guidelines for cardiac monitoring of children during and after anthracycline therapy: report of the Cardiology Committee of the Childrens Cancer Study Group. Pediatrics 1992;89:942-9. [PubMed]
- Nousiainen T, Jantunen E, Vanninen E, et al. Natriuretic peptides as markers of cardiotoxicity during doxorubicin treatment for non-Hodgkin's lymphoma. Eur J Haematol 1999;62:135-41. [Crossref] [PubMed]
- Armstrong GT, Plana JC, Zhang N, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol 2012;30:2876-84. [Crossref] [PubMed]
- Armstrong GT, Joshi VM, Ness KK, et al. Comprehensive Echocardiographic Detection of Treatment-Related Cardiac Dysfunction in Adult Survivors of Childhood Cancer: Results from the St. Jude Lifetime Cohort Study. J Am Coll Cardiol 2015;65:2511-22. [Crossref] [PubMed]
- Cheuk DK, Sieswerda E, van Dalen EC, et al. Medical interventions for treating anthracycline-induced symptomatic and asymptomatic cardiotoxicity during and after treatment for childhood cancer. Cochrane Database Syst Rev 2016.CD008011. [PubMed]
- Sieswerda E, Postma A, van Dalen EC, et al. Late Effects of Childhood Cancer The Dutch Childhood Oncology Group guideline for follow-up of asymptomatic cardiac dysfunction in childhood cancer survivors. Ann Oncol 2012;23:2191-8. [Crossref] [PubMed]
- Yusuf S, Pitt B, Davis CE, et al. SOLVD Investigators Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992;327:685-91. [Crossref] [PubMed]
- Lipshultz SE, Lipsitz SR, Sallan SE, et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol 2002;20:4517-22. [Crossref] [PubMed]
- Silber JH, Cnaan A, Clark BJ, et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol 2004;22:820-8. [Crossref] [PubMed]
- van Dalen EC, van der Pal HJ, van den Bos C, et al. Treatment for asymptomatic anthracycline-induced cardiac dysfunction in childhood cancer survivors: the need for evidence. J Clin Oncol 2003;21:3377; author reply 3377-8. [Crossref] [PubMed]
- van Dalen EC, van den Brug M, Caron HN, et al. Anthracycline-induced cardiotoxicity: comparison of recommendations for monitoring cardiac function during therapy in paediatric oncology trials. Eur J Cancer 2006;42:3199-205. [Crossref] [PubMed]
- Shankar SM, Marina N, Hudson MM, et al. Cardiovascular Disease Task Force of the Children's Oncology Group. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics 2008;121:e387-96. [Crossref] [PubMed]


