
Immune checkpoint inhibitors in esophagogastric cancer: still a long way to go
Immune checkpoint inhibitors (ICIs) targeting the programmed death 1/programmed death-ligand 1 (PD1/PD-L1) pathway have revolutionized treatment for various cancers. Evasion of the immune system is one of the hallmarks of cancer and chronic inflammation can facilitate cancer progression. Targeting gastrointestinal malignancies, a group of cancers that are, in part, inflammation-driven, is an appealing strategy but, with the exception of microsatellite unstable cancers (MSI-H), most patients do not benefit from ICI monotherapy (1-3). For patients with esophageal, gastroesophageal junction and gastric cancer (EGC), pembrolizumab is approved for PD-L1+ gastric or gastroesophageal junction adenocarcinoma after failure of at least two prior lines of therapy. The efficacy though is far from ideal. As shown in Table 1, data from key anti-PD1/PD-L1 monotherapy studies—with the exception of KEYNOTE-061 (conducted in the second-line setting in PD-L1+ disease) and the small EGC cohorts in KEYNOTE-028 and KEYNOTE-012 studies (including PD-L1+ esophageal carcinoma and gastric cancer, respectively) (4,5,7)—demonstrate that the efficacy of ICI in patients with advanced, heavily pre-treated disease is moderate at best. More importantly, ICI monotherapy does not appear better that single-agent chemotherapy in either the second- or third-line setting.
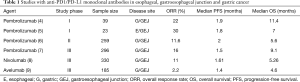
Full table
How can we improve the efficacy of ICI in EGC cancer? Janjigian and colleagues provide one possible answer in the CheckMate 032 study (10). The investigators hypothesized that dual ICIs with the anti-PD1 monoclonal antibody, nivolumab, plus the anti-cytotoxic T-cell lymphocyte antigen 4 (CTLA4) antibody, ipilimumab, can be more effective than anti-PD1 monotherapy. One hundred sixty patients with advanced, pretreated EGC were randomly assigned to nivolumab 3 mg/kg (NIVO3) every 2 weeks, nivolumab 1 mg/kg plus ipilimumab 3 mg/kg (NIVO1 + IPI3) every 3 weeks for four cycles; or nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (NIVO3 + IPI1) every 3 weeks for four cycles. All combination regimens were followed by NIVO3 every 2 weeks. Fifteen percent of the patients had esophageal primary and 79% had received at least 2 prior lines of therapy. The primary endpoint was overall response rate (ORR). The ORR was 12% with NIVO3, 24% with NIVO1+IPI3, and 8% with NIVO3 + IPI1. The median progression-free survival (PFS) and overall survival (OS) were 1.4/6.2, 1.4/6.9, and 1.6/4.8 months, in the NIVO3, NIVO1 + IPI3, and NIVO3 + IPI1 groups, respectively. As expected, grade 3 and 4 treatment-related adverse events (TRAEs) were more common in the NIVO1 + IPI3 and NIVO3 + IPI1 arms (47% and 27%, respectively compared to 17% in NIVO3 alone arm). Even though ORR appears higher with NIVO1 + IPI3, the long-term outcomes are similar to NIVO3 (12-month OS of 35% vs. 39%) and not much different compared to other ICI monotherapy (6,8,9). This can be related to the toxicity profile of NIVO1 + IPI3 (serious TRAEs, and TRAE leading to discontinuation in 43% and 20% of patients, respectively) that may easily decompensate a patient with advanced EGC.
How do we move forward? Perhaps the timing of introduction of ICI is not ideal. In KEYNOTE-059, the ORR was higher in patients receiving pembrolizumab as third vs. fourth line treatment (9). Further, in treatment-naïve patients with PD-L1+ tumors, the ORR with single-agent pembrolizumab was 26% in KEYNOTE-059 (Cohort 3) and the median survival was not reached (11,12). In addition, pembrolizumab plus cisplatin and 5-fluorouracil in the first-line setting (KEYNOTE-059/Cohort 2) resulted in an ORR of 60% (73% for PD-L1+ tumors); the median OS was 14 months for the 25 patients enrolled in this cohort (12,13). Multiple studies are now evaluating upfront chemoimmunotherapy and dual ICIs in patients with advanced EGC compared to chemotherapy alone as well as for treatment of patients with EGC in the localized setting. The second question raised is whether an anti-CTLA4 molecule together with an anti-PD1/PD-L1 drug is the best combination in this setting. There are multiple other immune checkpoint agents (immune agonists like OX40 and CD137 or antagonists such as LAG-3 and TIM-3) that can potentially add on to the activity of existing ICIs. Building into the paradigm of basket trials with mutation-specific targeted agents, the phase II Fast Real-time Assessment of Combination Therapies in Immuno-ONcology (FRACTION) study is designed to rapidly evaluate new combinations of strategies (14). In this study, patients who do not respond to the assigned treatment have the option to start a new regimen.
Finally, how can we best select patients for treatment with ICIs? The only approved biomarker is PD-L1 by immunohistochemistry (22C3 clone); the combined proportional score (CPS, defined as staining of cancer and contiguous mononuclear cells) should be >1 for treatment with pembrolizumab. In KEYNOTE-059, the ORR in PD-L1+ tumors (i.e., CPS >1) was 15.5% vs. 6.4% in PD-L1− tumors (6) while in a post hoc analysis of KEYNOTE-061, in patients with CPS>10, the median OS was 10.4 months with pembrolizumab (vs. 8 months in the paclitaxel arm; HR =0.64) (7). What is interesting though is that PD-L1 positivity can differ depending on the timing of testing (15,16). Further, the discriminatory activity of PD-L1 positivity is not consistent between studies including the study by Janjigian et al., with the caveat that different antibody clones are used (8-10). In KEYNOTE-059, T-cell inflamed tumors based on gene expression profiling had a higher probability of response and longer PFS; a CPS >20 was associated with a high T-cell inflamed score (6). In KEYNOTE-012, an interferon-related gene signature was not predictive of response (4). High tumor mutation burden (TMB) has been proposed as a predictive biomarker for response to ICIs in multiple cancers (17); about half of patients with esophageal adenocarcinoma can have a mutagenic signature based on whole-genome sequencing characterized by high TMB (18) and indeed, high TMB (>10/Mb) appears to predict long-term benefit from ICIs in retrospective studies (19,20).
In summary, Janjigian et al. are to be congratulated for the successful completion of a very challenging trial. We need to better define the patient population that can benefit the most from ICIs in esophageal or gastric cancer, the timing of ICI introduction to their care, and the best combination strategy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Zhang H, Xu X. Mutation-promoting molecular networks of uncontrolled inflammation. Tumour Biol 2017;39:1010428317701310. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717-26. [Crossref] [PubMed]
- Doi T, Piha-Paul SA, Jalal SI, et al. Safety and Antitumor Activity of the Anti-Programmed Death-1 Antibody Pembrolizumab in Patients With Advanced Esophageal Carcinoma. J Clin Oncol 2018;36:61-7. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RW, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 2018;4:e180013. [Crossref] [PubMed]
- Shitara K, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392:123-33. [Crossref] [PubMed]
- Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461-71. [Crossref] [PubMed]
- Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase 3, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment for patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol 2018;29:2052-60. [Crossref] [PubMed]
- Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol 2018;36:2836-44. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RW, et al. KEYNOTE-059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. J Clin Oncol 2017;35:abstr 4003.
- Wainberg ZA, Jalal S, Muro K, et al. LBA28_PRKEYNOTE-059 Update: Efficacy and safety of pembrolizumab alone or in combination with chemotherapy in patients with advanced gastric or gastroesophageal (G/GEJ) cancer. Ann Oncol 2017;28:mdx440.020-mdx440.020.
- Bang YJ, Muro K, Fuchs CS, et al. KEYNOTE-059 cohort 2: Safety and efficacy of pembrolizumab (pembro) plus 5-fluorouracil (5-FU) and cisplatin for first-line (1L) treatment of advanced gastric cancer. J Clin Oncol 2017;35:abstr 4012.
- Simonsen KL, Fracasso PM, Bernstein SH, et al. The Fast Real-time Assessment of Combination Therapies in Immuno-ONcology (FRACTION) program: innovative, high-throughput clinical screening of immunotherapies. Eur J Cancer 2018;103:259-66. [Crossref] [PubMed]
- Yang JH, Kim H, Roh SY, et al. Discordancy and changes in the pattern of programmed death ligand 1 expression before and after platinum-based chemotherapy in metastatic gastric cancer. Gastric Cancer 2019;22:147-54. [Crossref] [PubMed]
- Shen JYC, Usher J, Samberg D, et al. PD-L1 and HER2 expression in gastric cancer (GC) patients (pts) using cell-free RNA (cfRNA). J Clin Oncol 2016;34:e15539. [Crossref]
- Goodman AM, Kato S, Bazhenova L, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther 2017;16:2598-608. [Crossref] [PubMed]
- Secrier M, Li X, de Silva N, et al. Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nature Genetics 2016;48:1131. [Crossref] [PubMed]
- Ku GY, Sanchez-Vega F, Chatila W, et al. Correlation of benefit from immune checkpoint inhibitors with next gen sequencing (NGS) profiles in esophagogastric cancer (EGC) patients. J Clin Oncol 2017;35:abstr 4025.
- Janjigian YY, Sanchez-Vega F, Jonsson P, et al. Genetic Predictors of Response to Systemic Therapy in Esophagogastric Cancer. Cancer Discov 2018;8:49-58. [Crossref] [PubMed]


