
Lung transplantation and beyond: continued challenges in the wake of significant progress
Since the first clinical lung transplant was successfully performed in 1983 (1), lung transplantation has evolved into a well-recognized therapy for patients with end-stage lung diseases. According to the most recent registry data from the International Society of Heart and Lung Transplantation (ISHLT) (2), the total number of lung transplants performed in the world continues to increase and is currently approximately 4,000 cases annually. Interestingly, however, most lung transplants are performed at a limited number of centers. One-third of all lung transplants are done at 14 transplant centers that each perform more than 50 cases per year. These centers are recognized as “high-volume transplant centers”.
In the August 2018 issue of the Journal of Thoracic and Cardiovascular Surgery, Balsara and colleagues (3) from Barnes Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri, a high-volume center as well as one of the most historic transplant institutions in United States (Tables 1,2), detail their single-institution experience with lung transplantation in 1,500 patients over a 30-year period and compare patient characteristics and outcomes before and after the introduction of the lung allocation score (LAS) in 2005 (4). Balsara and colleagues very nicely demonstrate improved long-term survival outcomes despite transplanting high-acuity patients more frequently in the post-LAS era. There are two additional findings from the study that I find particularly intriguing. The first is that the patients in the post-LAS era had a higher incidence of severe primary graft dysfunction (PGD) as compared with those in the pre-LAS era (grade 3 PGD, 31% versus 22%), but still had improved long-term survival and freedom from bronchiolitis obliterans syndrome (BOS). The second is that the Washington University group performed almost exclusively double-lung transplants (96%) in the post-LAS era and strongly advocates that double-lung transplant should be the standard for lung transplantation.
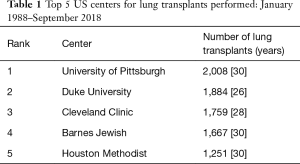
Full table
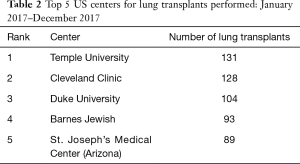
Full table
With regards to the first finding—an increased incidence of PGD in the post-LAS era—while I support the authors’ point that we should prioritize research to understand the drivers underlying PGD and new therapeutic strategies to minimize its occurrence, lung transplant surgeons also should be mindful that improvements to the surgical techniques used for lung transplant may reduce PGD and/or BOS; however, the standard surgical techniques for lung transplant have basically remained unchanged for the last two decades, and only a few modifications to these techniques have improved long-term outcomes. One of these few exceptions is direct bronchial artery revascularization (BAR) (5-7). As the only solid organ transplant procedure without surgical connection of all major viable arteries to the allograft, the conducting airways, from the main bronchus to the terminal respiratory bronchioles, in transplanted lung grafts are at risk for complications. Recent, robust, basic-science evidence demonstrated that compromised microvasculature and poor perfusion in transplanted lungs, resulting from a lack of bronchial arterial circulation at the time of transplant, trigger BOS (8-10). Damaged microvasculature and poor perfusion are major determinants of the development of organ graft failure, which all transplant physicians must bear in mind when transplanting solid organs (11).
When pioneers in the lung transplant field reported their initial experiences with BAR, with the goal of using BAR to reduce the incidence of anastomotic complications in the airways, it was well received. In contrast, their attempts to show beneficial effects of restoring the bronchial arterial circulation on airway healing and long-term survival appeared to fail due to insufficient data regarding BOS and the small number of patients enrolled in their study (12,13). However, since the initial publications in the late 1990s, Dr. Petterson and his team at the Cleveland Clinic have steadily accumulated experience with BAR, and their most recent report, published in 2015, shows more promising data. They demonstrated that BAR delayed the onset of BOS and improved long-term survival (14). Major limitations of BAR are that it is a very complicated technique and that it is difficult to teach. As a result, Petterson’s results have poor generalizability to all lung transplant surgeons, even at high-volume centers. Nonetheless, their focus on optimizing the microvasculature in lung grafts should remain a central focus in the field while attempting to circumvent the complexity of BAR. Lung transplant surgeons need to strive for alternatives to sacrificing the bronchial arterial circulation, and our team at Temple University is aggressively working on this (15,16).
The second message from Balsara and colleagues—that double-lung transplantation should be the standard approach in the field—needs to be carefully interpreted. The patients in the Washington University study cohort were relatively young (48 years in the pre-LAS era and 50 years in the post-LAS era). In the United States, there has been a steadily increase in the number of lung transplant recipients who are older than 70 years of age (17). Several reports suggest that double-lung transplant is not necessarily a superior option in elderly patients or patients with morbid obesity or other comorbidities (18,19). In addition, in aging populations many patients have multiple diseases characterized by acceleration of the normal ageing process while common mechanisms of accelerated ageing including oxidative stress, telomere shortening, or cellular senescence are shared between these diseases (20). Cardiovascular disease is one such chronic disease associated with accelerated aging and has been increasingly seen in elderly lung transplant candidates (21). Historically, lung transplantation was not considered for older patients with major cardiovascular comorbidities. However, recently due to remarkable progress in minimally invasive, interventional therapies, such as percutaneous coronary intervention (PCI) and transcatheter aortic valve replacement (TAVR), physicians caring for elderly patients have acquired the ability to treat co-existing problems, enabling some elderly patients with cardiovascular comorbidities become potentially viable candidates for lung transplant.
While the data from Washington University demonstrate ways to obtain the best outcomes in the current, LAS era, I would stress that it is also important to think about how to cope with challenges that lung transplant physicians are likely to encounter. Currently in the United States, the expected remaining-years-of-life for a person reaching the age of 65, which most transplant centers consider to be the cut-off age to become a viable lung transplant candidate, is 18 years for males and 20 years for females (22). While continuing to prioritize long-term survival as the primary goal for all patients receiving a lung transplant, we also need to cope with rising life expectancy in many societies. Double-lung transplant may not be a good option for patients older than 70 years of age with prior PCI or TAVR. While donor-recipient age matching should be considered to optimize utilization of the donor pool, we do not know if utilizing marginal, age-matched donors will achieve acceptable outcomes. Additionally, we need to define specific goals for lung transplant in patients over 70 years old or with diseases associated with accelerated aging. For example, should 1-year survival rather than 5-year survival serve as our benchmark for these patients?
High-volume transplant centers are privileged to spearhead future directions in lung transplantation by pushing the envelope while demonstrating consistently improving transplant outcomes as the leaders of the field. Indeed, in high-volume centers, there has been a paradigm shift toward performing lung transplant in patients with LASs in the highest tertile and a softening of attitudes toward the use of support before lung transplantation, such as prior mechanical ventilation (MV) and extracorporeal lung support (ECLS) including extracorporeal membrane oxygenation (ECMO) (23). This is, in part, because organs from available donors are preferentially directed toward the sickest patients and the centers caring for them, as noted in Balsara’s article (3). Given that the Washington University group performed only two cases with ECMO bridging to lung transplant, out of 1,500 patients in their study, they may be more selective against prior mechanical support in lung transplant candidates. We recently demonstrated that MV or MV + ECMO as a bridge to lung transplantation did not lead to statistically significant differences in most postoperative complications or in overall survival, as compared with transplant recipients without prior mechanical support (23). Of note, the use of MV with ECMO as a bridge to transplantation significantly increased survival as compared with MV alone. Hoetzenecker and colleagues from Toronto also recently reported their unique experiences using ECLS technology to bridge patients to lung transplantation. Using a variety of available ECLS devices and modes, they concluded that ECLS is clearly an effective tool to bridge critically ill patients to lung transplant, and that ECLS can be individualized according to each patient’s needs, thus minimizing morbidity and optimizing outcomes following lung transplantation (24). Indeed, accumulating evidence supports using ECMO and ECLS to treat lung failure and support patients before and after lung transplantation, and the success of ECLS in lung transplantation sheds a new light on its expanding use toward long-term artificial respiratory support for advanced lung failure (25). In the future, long-term artificial respiratory support or artificial lungs might become an alternative to lung transplantation, replacing the need for donor lungs with a fully functional, man-made device incorporated into the respiratory and circulatory systems. The lung transplant surgeons at high-volume centers need to move the field forward toward this promising possibility.
In conclusion, Balsara and colleagues (3) provide an excellent and useful institutional experience incorporating current lung transplantation paradigms from one of the highest volume transplant centers in United States and highlight the challenges encountered in the field. Further studies and research should be encouraged to overcoming the current major limitations in clinical lung transplantation.
AcknowledgementsOther Section
None.
FootnoteOther Section
Conflicts of Interest: The author has no conflicts of interest to declare.
ReferencesOther Section
- Toronto Lung Transplant Group. Unilateral lung transplantation for pulmonary fibrosis. N Engl J Med 1986;314:1140-5. [Crossref] [PubMed]
- Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: Multiorgan Transplantation. J Heart Lung Transplant 2018;37:1169-83. [Crossref] [PubMed]
- Balsara KR, Kruknick AS, Bell JM, et al. A single-center experience of 1500 lung transplant patients. J Thorac Cardiovasc Surg 2018;156:894-905.e3. [Crossref] [PubMed]
- Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant 2006;6:1212-27. [Crossref] [PubMed]
- Couraud L, Baudet E, Martigne C, et al. Bronchial revascularization in double-lung transplantation: a series of 8 patients. Bordeaux Lung and Heart Lung Transplant Group. Ann Thorac Surg 1992;53:88-94. [Crossref] [PubMed]
- Pettersson G, Arendrup H, Mortensen SA, et al. Early experience of double-lung transplantation with bronchial artery revascularization using mammary artery. Eur J Cardiothorac Surg 1994;8:520-4. [Crossref] [PubMed]
- Nørgaard MA, Olsen PS, Svendsen UG, et al. Revascularization of the bronchial arteries in lung transplantation: an overview. Ann Thorac Surg 1996;62:1215-21. [Crossref] [PubMed]
- Jiang X, Khan MA, Tian W, et al. Adenovirus-mediated HIF-1 alpha gene transfer promotes repair of mouse airway allograft microvasculature and attenuates chronic rejection. J Clin Invest 2011;121:2336-49. [Crossref] [PubMed]
- Dhillon GS, Zamora MR, Roos JE, et al. Lung transplant airway hypoxia: a diathesis to fibrosis? Am J Respir Crit Care Med 2010;182:230-6. [Crossref] [PubMed]
- Kraft BD, Suliman HB, Colman EC, et al. Hypoxic gene expression of donor bronchi linked to airway complications after lung transplantation. Am J Respir Crit Care Med 2016;193:552-60. [Crossref] [PubMed]
- Jiang X, Sung YK, Tian W, et al. Graft microvasculature disease in solid organ transplantation. J Mol Med (Berl) 2014;92:797-810. [Crossref] [PubMed]
- Baudet EM, Dromer C, Dubrez J, et al. Intermediate-term results after en bloc double-lung transplantation with bronchial artery revascularization. Bordeaux Lung and Heart Lung Transplant Group. J Thorac Cardiovasc Surg 1996;112:1292-9; discussion 1299-300. [Crossref] [PubMed]
- Nørgaard MA, Andersen CB, Petterson G. Does bronchial artery revascularization influence results concerning bronchiolitis obliterans syndrome and/or obliterative bronchiolitis after lung transplantation? Eur J Cardiothorac Surg 1998;14:311-8. [Crossref] [PubMed]
- Tong MZ, Johnston DR, Petterson GB. The role of bronchial artery revascularization in lung transplantation. Thorac Surg Clin 2015;25:77-85. [Crossref] [PubMed]
- Tanaka Y, Noda K, Isse K, et al. A novel dual ex vivo lung perfusion technique improves immediate outcomes in an experimental model of lung transplantation. Am J Transplant 2015;15:1219-30. [Crossref] [PubMed]
- Shigemura N, Tane S, Noda K. The bronchial arterial circulation in lung transplantation: Bedside to bench to bedside, and beyond. Transplantation 2018;102:1240-9. [Crossref] [PubMed]
- Hayanga AJ, Aboagye JK, Hayanga H, et al. Contemporary analysis of early outcomes after lung transplantation in the elderly using a national registry. J Heart Lung Transplant 2015;34:182-8. [Crossref] [PubMed]
- Gulack BC, Ganapathi AM, Speicher PJ, et al. What is the optimal transplant for older patients with idiopathic pulmonary fibrosis? Ann Thorac Surg 2015;100:1826-33. [Crossref] [PubMed]
- Gries CJ, Bhadriraju S, Edelman JD, et al. Obese patients with idiopathic pulmonary fibrosis have a higher 90-day mortality risk with bilateral lung transplantation. J Heart Lung Transplant 2015;34:241-6. [Crossref] [PubMed]
- Barnes PJ. Mechanisms of development of multimorbidity in the elderly. Eur Respir J 2015;45:790-806. [Crossref] [PubMed]
- Koprivanac M, Budev MM, Yun JJ, et al. How important is coronary artery disease when considering lung transplant candidates? J Heart Lung Transplant 2016;35:1453-61. [Crossref] [PubMed]
- Wigfield CH, Buie V, Onsager D. Age in lung transplantation: factors related to outcomes and other considerations. Curr Pulmonol Rep 2016;5:152-8. [Crossref] [PubMed]
- Hayanga AJ, Du AL, Joubert K, et al. Mechanical ventilation and extracorporeal membrane oxygenation as a bridging strategy to lung transplantation: Significant gains in survival. Am J Transplant 2018;18:125-35. [Crossref] [PubMed]
- Hoetzenecker K, Donahoe L, Yeung JC, et al. Extracorporeal life support as a bridge to lung transplantation – experience of a high-volume transplant center. J Thorac Cardiovasc Surg 2018;155:1316-1328.e1. [Crossref] [PubMed]
- Naito N, Keith C, Toyoda Y, et al. Artificial lungs for lung failure. J Am Coll Cardiol 2018;72:1640-52. [Crossref] [PubMed]


